1st U.S. case of COVID omicron variant confirmed in California

A person in California who had been vaccinated against COVID-19 became the first in the U.S. to have an identified case of the omicron variant; the White House announced Wednesday as scientists continue to study the risks posed by the new virus strain. Dr. Anthony Fauci told reporters the person was a traveler who returned from South Africa on November 22 and tested positive on November 29. Fauci said the person was vaccinated but had not received a booster shot and was experiencing “mild symptoms.” The Biden administration moved late last month to restrict travel from Southern Africa, where the variant was first identified and had been widespread. Clusters of cases have also been identified in about two dozen other nations. “We knew that it was just a matter of time before the first case of omicron would be detected in the United States,” Fauci said. He said the person was improving and added, “I think what’s happening now is another example of why it’s important for people to get vaccinated. But also boosting. Boosting is very important.” Officials said they had contacted everyone who had close contact with the person, and they had all tested negative. Genomic sequencing was conducted at the University of California, San Francisco, and the sequence was confirmed by the Centers for Disease Control and Prevention. The Centers for Disease Control and Prevention is taking steps to tighten U.S. testing rules for travelers from overseas, including requiring a test for all travelers within a day of boarding a flight to the U.S. regardless of vaccination status. It was also considering mandating post-arrival testing. Officials said those measures would only “buy time” for the country to learn more about the new variant and to take appropriate precautions, but that given its transmissibility, its arrival in the U.S. was inevitable. Much remains unknown about the new variant, including whether it is more contagious than previous strains, whether it makes people more seriously ill, and whether it can thwart the vaccine. Fauci, the top U.S. infectious disease expert, said more would be known about the omicron strain in two to four weeks as scientists grow and test lab samples of the virus. California’s Department of Public Health credited the state’s “large-scale testing and early detection systems” for identifying the case. “We recognize that everyone is exhausted, and the news of a new variant can be overwhelming. It is important that we collectively focus on the things we know prevent the spread of COVID-19 and its variants,” state public health officials said in a statement. The announcement of the first U.S. case comes before President Joe Biden plans to outline his strategy on Thursday to combat the virus over the winter. Biden has tried to quell alarm over the omicron variant, saying it was a cause for concern but “not a cause for panic.” Biden and public health officials have grown more urgent in their pleas for more Americans to get vaccinated — and for those who have been vaccinated to get booster shots to maximize their protection against the virus. Republished with the permission of the Associated Press.
Merck asks U.S. FDA to authorize promising anti-COVID pill

Drugmaker Merck asked U.S. regulators Monday to authorize its pill for treating COVID-19 in what would add an entirely new and easy-to-use weapon to the world’s arsenal against the pandemic. If cleared by the Food and Drug Administration — a decision that could come in a matter of weeks — it would be the first pill shown to treat the illness. All other FDA-backed treatments against COVID-19 require an IV or injection. An antiviral pill that people could take at home to reduce their symptoms and speed recovery could prove groundbreaking, easing the crushing caseload on U.S. hospitals and helping to curb outbreaks in poorer countries with weak health care systems. It would also bolster the two-pronged approach to the pandemic: treatment, by way of medication, and prevention, primarily through vaccinations. The FDA will scrutinize company data on the safety and effectiveness of the drug, molnupiravir, before rendering a decision. Merck and its partner Ridgeback Biotherapeutic said they specifically asked the agency to grant emergency use for adults with mild-to-moderate COVID-19 who are at risk for severe disease or hospitalization. That is roughly the way COVID-19 infusion drugs are used. “The value here is that it’s a pill so you don’t have to deal with the infusion centers and all the factors around that,” said Dr. Nicholas Kartsonis, a senior vice president with Merck’s infectious disease unit. “I think it’s a very powerful tool to add to the toolbox.” The company reported earlier this month that the pill cut hospitalizations and deaths by half among patients with early symptoms of COVID-19. The results were so strong that independent medical experts monitoring the trial recommended stopping it early. Side effects were similar between patients who got the drug and those in a testing group who received a dummy pill. But Merck has not publicly detailed the types of problems reported, which will be a key part of the FDA’s review. Top U.S. health officials continue to push vaccinations as the best way to protect against COVID-19. “It’s much, much better to prevent yourself from getting infected than to have to treat an infection,” Dr. Anthony Fauci said while discussing Merck’s drug last week. Still, some 68 million eligible Americans remain unvaccinated, underscoring the need for effective drugs to control future waves of infection. The prospect of a COVID-19 pill comes amid other encouraging signs: New cases per day in the U.S. have dropped below 100,000 on average for the first time in over two months, and deaths are running at about 1,700 a day, down from more than 2,000 three weeks ago. Also, the average number of vaccinations dispensed per day has climbed past 1 million, an increase of more than 50% over the past two weeks, driven by the introduction of booster shots and workplace vaccine requirements. Still, health authorities are bracing for another possible surge as cold weather drives more people indoors. Since the beginning of the pandemic, health experts have stressed the need for a convenient pill. The goal is for something similar to Tamiflu, the 20-year-old flu medication that shortens the illness by a day or two and blunts the severity of symptoms like fever, cough, and stuffy nose. Three FDA-authorized antibody drugs have proved highly effective at reducing COVID-19 deaths, but they are expensive, hard to produce, and require specialty equipment and health professionals to deliver. Assuming FDA authorization, the U.S. government has agreed to buy enough of the pills to treat 1.7 million people, at a price of roughly $700 for each course of treatment. That’s less than half the price of the antibody drugs purchased by the U.S. government — over $2,000 per infusion — but still more expensive than many antiviral pills for other conditions. Merck’s Kartsonis said in an interview that the $700 figure does not represent the final price for the medication. “We set that price before we had any data, so that’s just one contract,” Kartsonis said. “Obviously we’re going to be responsible about this and make this drug as accessible to as many people around the world as we can.” Kenilworth, New Jersey-based Merck has said it is in purchase talks with governments around the world and will use a sliding price scale based on each country’s economic means. Also, the company has signed licensing deals with several Indian generic drugmakers to produce low-cost versions of the drug for lower-income countries. Several other companies, including Pfizer and Roche, are studying similar drugs and are expected to report results in the coming weeks and months. AstraZeneca is also seeking FDA authorization for a long-acting antibody drug intended to provide months of protection for patients who have immune-system disorders and do not adequately respond to vaccination. Some experts predict various COVID-19 therapies eventually will be prescribed in combination to better protect against the worst effects of the virus. Republished with the permission of the Associated Press.
Merck says experimental pill cuts worst effects of COVID-19

Drugmaker Merck said Friday that its experimental COVID-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus and that it would soon ask health officials in the U.S. and around the world to authorize its use. If cleared, the drug would be the first pill shown to treat COVID-19, a potentially major advance in efforts to fight the pandemic. All COVID-19 therapies now authorized in the U.S. require an IV or injection. Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received the drug, called molnupiravir, within five days of COVID-19 symptoms had about half the rate of hospitalization and death as patients who received a dummy pill. The study tracked 775 adults with mild-to-moderate COVID-19 who were considered at higher risk for severe disease due to health problems such as obesity, diabetes, or heart disease. Among patients taking molnupiravir, 7.3% were either hospitalized or died at the end of 30 days, compared with 14.1% of those getting the dummy pill. There were no deaths in the drug group after that time period compared with eight deaths in the placebo group, according to Merck. The results were released by the company and have not been peer-reviewed by outside experts, the usual procedure for vetting new medical research. Merck said it plans to present them at a future medical meeting. An independent group of medical experts monitoring the trial recommended stopping it early because the interim results were so strong. That is typical when early results so clearly show treatment works that there is no need for further testing before applying for authorization. Company executives said they plan to submit the data for review by the Food and Drug Administration in the coming days. Once the submission is complete, the FDA could make a decision within weeks — and, if approved, the drug could be on the market soon after. “It exceeded what I thought the drug might be able to do in this clinical trial,” said Dr. Dean Li, vice president of Merck Research Laboratories. “When you see a 50% reduction in hospitalization or death, that’s a substantial clinical impact.” Side effects were reported by both groups in the Merck trial, but they were slightly more common among the group that received a dummy pill. The company did not specify the problems. Patients take the pill twice a day for five days to complete a course of treatment. Earlier study results showed the drug did not benefit patients who were already hospitalized with severe disease. The U.S. has approved one antiviral drug, remdesivir, specifically for COVID-19, and allowed emergency use of three antibody therapies that help the immune system fight the virus. But all the drugs have to be given by IV or injection at hospitals or medical clinics, and supplies have been stretched by the latest surge of the delta variant. Health experts, including the top U.S. infectious disease expert Dr. Anthony Fauci have long called for a convenient pill that patients could take when COVID-19 symptoms first appear, much the way the standard flu medication Tamiflu helps fight influenza. Such medications are seen as key to controlling future waves of infection and reducing the impact of the pandemic. Merck’s pill works by interfering with the coronavirus’s ability to copy its genetic code and reproduce itself. It has shown similar activity against other viruses. The U.S. government has committed to purchase 1.7 million doses of the drug if it is authorized by the FDA. Merck has said it can produce 10 million doses by the end of the year and has contracts with governments worldwide. The company has not announced prices. Several other companies, including Pfizer and Roche, are studying similar drugs that could report results in the coming weeks and months. Merck had planned to enroll more than 1,500 patients in its late-stage trial before the independent board stopped it early. The results reported Friday included patients enrolled across Latin America, Europe, and Africa. Executives estimated about 10% of patients studied were from the U.S. Republished with the permission of the Associated Press.
U.S. health officials call for booster shots against COVID-19
U.S. health officials Wednesday announced plans to dispense COVID-19 booster shots to all Americans to shore up their protection amid the surging delta variant and signs that the vaccines’ effectiveness is slipping. The plan, as outlined by the chief of the Centers for Disease Control and Prevention and other top health authorities, calls for an extra dose eight months after people get their second shot of the Pfizer or Moderna vaccine. The doses could begin the week of Sept. 20. “Our plan is to protect the American people, to stay ahead of this virus,” CDC Director Dr. Rochelle Walensky said as the agency cited a raft of studies suggesting that the vaccines are losing ground while the highly contagious variant spreads. People who received the single-dose Johnson & Johnson vaccine will also probably need extra shots, health officials said. But they said they are waiting for more data. Officials said that before any booster program starts up, the Food and Drug Administration and a CDC advisory panel would need to evaluate the safety and effectiveness of an extra dose. “We have a responsibility to give the maximum amount of protection,” President Joe Biden said at the White House. He added that extra doses are also “the best way to protect ourselves from new variants that could arise.” The announcement came the same day the Biden administration said it would require nursing homes to mandate vaccinations for staffers in order to continue receiving federal funds. Hundreds of thousands of nursing home workers remain unvaccinated, despite the heightened risk of fatal infections among elderly residents. Officials said it is “very clear” that the vaccines’ protection against infections wanes over time, and they noted the worsening picture in Israel, which has seen a rise in severe cases, many of them in people already inoculated. They said the U.S. needs to get out ahead of the problem before it takes a more lethal turn here and starts leading to increasing hospitalizations and deaths among the vaccinated. Dr. Anthony Fauci, the government’s foremost expert on COVID-19, said one of the key lessons of the coronavirus is that it’s better to “stay ahead of it than chasing after it.” The first boosters would go to people in high-priority groups that received the initial U.S. vaccinations: nursing home residents, health workers, and those with underlying health conditions. Health officials are likely to recommend that the booster be the same brand of vaccine that people received initially. Dr. Mark Mulligan of NYU’s Langone Health center welcomed the announcement, saying: “Part of leadership is being able to see around the corner and make hard decisions without having all the data. It seems to me that’s what they’re doing here.” Top scientists at the World Health Organization bitterly objected to the U.S. plan, noting that poor countries are not getting enough vaccine for their initial rounds of shots. “We’re planning to hand out extra life jackets to people who already have life jackets, while we’re leaving other people to drown without a single life jacket,” said Dr. Michael Ryan, the WHO’s emergencies chief. The organization’s top scientist, Dr. Soumya Swaminathan, said the evidence does not show boosters are needed for everyone, and she warned that leaving billions of people in the developing world unvaccinated could foster the emergence of new variants and result in “even more dire situations.” U.S. Surgeon General Vivek Murthy rejected the notion that the U.S. must choose between “America and the world,” saying: “We clearly see our responsibility to both.” White House officials noted that the U.S. has donated 115 million doses to 80 countries, more than all other nations combined. They said the U.S. has plenty of vaccine to dispense boosters to its own population. Israel is already offering booster shots to people over 50. And European regulators are looking into the idea. Last week, U.S. health officials recommended a third shot for some people with weakened immune systems, such as cancer patients and organ transplant recipients. Offering boosters to all Americans would be a major expansion of what is already the biggest vaccination campaign in U.S. history. Nearly 200 million Americans have received at least one shot. Some experts have expressed concern that calling for boosters would undermine the public health message — and reinforce opposition to the vaccine — by raising more doubts in the minds of people already skeptical about the shots’ effectiveness. As for why the vaccines appear to be less effective over time at stopping infections, there are indications that the body’s immune response to the shots fades, as it does with other inoculations. But also, the vaccines simply may not protect against the delta variant as well as they do against the original virus. Scientists are still trying to answer the question. Officials said the eight-month timeframe was a judgment call about when vaccine protection against severe illness might fall, based on the direction of the current data. “There’s nothing magical about this number,” the surgeon general said. Nearly 20 months into the outbreak, the scourge has killed 620,000 Americans. Just weeks after the president declared the country’s “independence” from COVID-19 on July Fourth, emergency rooms in parts of the South and West are overloaded again, and cases are averaging nearly 140,000 per day, quadrupling in just a month. In making its announcement, the CDC released a number of studies conducted during the delta surge that suggest that the vaccines remain highly effective at keeping Americans out of the hospital but that their ability to prevent infection is dropping markedly. One of the studies looked at reported COVID-19 infections in residents of nearly 15,000 nursing homes and other long-term care facilities. It found that the effectiveness of the Pfizer and Moderna vaccines against infection fell from about 74% in March, April, and early May to 53% in June and July. The study examined all COVID-19 infections, with or without symptoms. The researchers said more work is needed to determine if there was a higher incidence of infections that resulted in
Anthony Fauci hopeful COVID vaccines get full OK by FDA within weeks
The U.S. government’s top infectious disease expert, Dr. Anthony Fauci, said Sunday that he was hopeful the Food and Drug Administration will give full approval to the coronavirus vaccine by month’s end and predicted the potential move will spur a wave of vaccine mandates in the private sector as well as schools and universities. The FDA has only granted emergency-use approval of the Pfizer, Moderna, and Johnson & Johnson vaccines, but the agency is expected to soon give full approval to Pfizer. The Biden administration has stated that the federal government will not mandate vaccinations beyond the federal workforce but is increasingly urging state and local governments as well as businesses to consider such mandates. Fauci, who is President Joe Biden’s chief medical adviser, said “mandates at the local level need to be done” to help curb the spread of the virus. “I hope — I don’t predict — I hope that it will be within the next few weeks. I hope it’s within the month of August,” Fauci said of FDA approval of the vaccine. “If that’s the case, you’re going to see the empowerment of local enterprises, giving mandates that could be colleges, universities, places of business, a whole variety, and I strongly support that. The time has come. … We’ve got to go the extra step to get people vaccinated.” Fauci’s comments come as the Biden administration is weighing what levers it can push to encourage more unvaccinated Americans to get their shots as the delta variant continues to surge through much of the United States. Biden recently approved rules requiring federal workers to provide proof of vaccination or face regular testing, mask mandates, and travel restrictions. Biden is also awaiting a formal recommendation from Defense Secretary Lloyd Austin on potentially requiring U.S. troops to get vaccinated. The administration has become more vocal in its support of vaccine mandates at a moment when high-profile companies have informed employees that coronavirus vaccination requirements are in the works, and some localities have adopted or are contemplating vaccine requirements to dine indoors. United Airlines informed its employees that they will need to be fully vaccinated by Oct. 25 or five weeks after the FDA grants full approval to one of the vaccines — whichever date comes first. Disney and Walmart have announced vaccine mandates for white-collar workers, and Microsoft, Google, and Facebook said they will require proof of vaccination for employees and visitors to their U.S. offices. Tyson Foods has also announced it will require all U.S. employees to get vaccinated by November. There’s also been pushback. The U.S. Supreme Court last week was asked to block a plan by Indiana University to require students and employees to get vaccinated against COVID-19. It’s the first time the high court has been asked to weigh in on a vaccine mandate and comes as some corporations, states, and cities are also contemplating or have adopted vaccine requirements for workers or even to dine indoors. Randi Weingarten, president of the American Federation of Teachers union, said on Sunday that she personally supports a vaccine mandate for educators. “As a matter of personal conscience, I think that we need to be working with our employers — not opposing them on vaccine mandates,” said Weingarten, who estimated about 90% of AFT members are already vaccinated. Dr. Francis Collins, director of the National Institutes of Health, on Sunday all but endorsed vaccine mandates, saying, “I celebrate when I see businesses deciding that they’re going to mandate that for their employees.” “Yes, I think we ought to use every public health tool we can when people are dying,” Collins said. Fauci and Weingarten spoke on NBC’s “Meet the Press,” and Collins appeared on ABC’s “This Week.” Republished with the permission of the Associated Press.
Anthony Fauci: More ‘pain and suffering’ ahead as COVID cases rise
Dr. Anthony Fauci warned Sunday that more “pain and suffering” is on the horizon as COVID-19 cases climb again and officials plead with unvaccinated Americans to get their shots. Fauci, the nation’s top infectious disease expert, also said he doesn’t foresee additional lockdowns in the U.S. because he believes enough people are vaccinated to avoid a recurrence of last winter. However, he said not enough are inoculated to “crush the outbreak” at this point. Fauci’s warning comes days after the Centers for Disease Control and Prevention changed course to recommend that even vaccinated people return to wearing masks indoors in parts of the U.S. where the delta variant is fueling infection surges. With the switch, federal health officials have cited studies showing vaccinated people can spread the virus to others. Most new infections in the U.S. continue to be among unvaccinated people. So-called breakthrough infections can occur in vaccinated people, and though the vast majority of those cause mild or no symptoms, the research shows they can carry about the same amount of the coronavirus as those who did not get the shots. “So we’re looking, not, I believe, to lockdown, but we’re looking to some pain and suffering in the future because we’re seeing the cases go up, which is the reason why we keep saying over and over again, the solution to this is get vaccinated, and this would not be happening,” Fauci said on ABC’s “This Week.” According to data through July 30 from Johns Hopkins University, the seven-day rolling average for daily new cases in the U.S. rose from 30,887 on July 16 to 77,827 on July 30. The seven-day rolling average for the country’s daily new deaths rose over the same period from 253 on July 16 to 358 on July 30, though death reports generally lag weeks after infections and even longer after hospitalizations. Currently, 58% of Americans 12 years and older are fully vaccinated, according to the CDC’s data tracker. However, people are “getting the message,” and more are rolling up their sleeves amid the threat of the delta variant, according to the director of the National Institutes of Health. Dr. Francis Collins said on CNN’s “State of the Union” that vaccinations are up 56% in the U.S. in the last two weeks. Louisiana, which has the most new cases per capita among states in the past 14 days, has seen vaccinations up threefold over that period, Collins said. “That’s what desperately needs to happen if we are going to get this delta variant put back in its place because right now it’s having a pretty big party in the middle of the country,” Collins said. Collins also said that even with the prevalence of the delta variant, the shots are working “extremely well” and reduce a person’s risk of serious illness and hospitalization “25-fold.” The guidance for vaccinated people to start wearing masks indoors again in certain places with worsening outbreaks, he said, is mostly meant to protect unvaccinated and immunocompromised people. The CDC has also recommended indoor mask-wearing for all teachers, staff, students, and visitors at schools nationwide, regardless of vaccination status. Republished with the permission of the Associated Press.
Alabama Public Health announces TikTok contest to encourage vaccinations
Alabama Public Health is starting to use some creative ways to encourage more Alabamians to get vaccinated. With a low vaccination rate across the state, only 30% of residents are fully vaccinated, leaders have had to find new ways to encourage vaccination. President Joe Biden recently called on younger Americans to get vaccinated and even has the star power to help. Singer Olivia Rodrigo recently met with Biden and Anthony Fauci to discuss ways to help the lagging vaccination rate in young people. According to their website, the Alabama Department of Public Health (ADPH) is sponsoring a TikTok contest for people between the ages of 13 and 29 to encourage vaccination against COVID-19 before the beginning of the school year. Contestants must submit a TikTok video showing themselves getting vaccinated and should include a message explaining, “This is why I got vaccinated.” ADHP will select four winners, and each will receive a $250 Visa gift card. The Pfizer vaccine was made available for children under 18 in May. Since then only 37 percent of residents age 12 to 17 have gotten at least one dose and only 38 percent of 18 to 29-year-olds have received at least one dose reported Politico. According to the Centers for Disease Control and Prevention, 56.2% of Americans have gotten at least one dose of the vaccine. The delta variant is much more contagious and most people who have complications from coronavirus are unvaccinated. COVID-19 cases have nearly tripled in the U.S. over two weeks.
Anthony Fauci, Rand Paul clash on virus origins, trade charges of lying

Dr. Anthony Fauci, the nation’s top infectious disease expert, angrily confronted Kentucky GOP Sen. Rand Paul on Tuesday in testimony on Capitol Hill, rejecting Paul’s insinuation that the U.S. helped fund research at a Chinese lab that could have sparked the COVID-19 outbreak. Paul suggested that Fauci had lied before Congress when in May, he denied that the National Institutes of Health funded so-called “gain of function” research — the practice of enhancing a virus in a lab to study its potential impact in the real world — at a Wuhan virology lab. U.S. intelligence agencies are currently exploring theories that an accidental leak from that lab could have led to the global pandemic. “I have not lied before Congress. I have never lied. Certainly not before Congress. Case closed,” Fauci told Paul before the Senate Health, Education, Labor, and Pensions Committee, saying a study the senator mentioned referenced a different sort of virus entirely from the one responsible for the coronavirus outbreak. “Senator Paul, you do not know what you’re talking about, quite frankly,” Fauci said. “And I want to say that officially. You do not know what you’re talking about.” He added, “If anybody is lying here, senator, it is you.” It was the latest in a series of clashes between Paul and Fauci over the origins of the virus that caused the global pandemic. Republished with the permission of the Associated Press.
U.S. COVID-19 cases rising again, doubling over three weeks
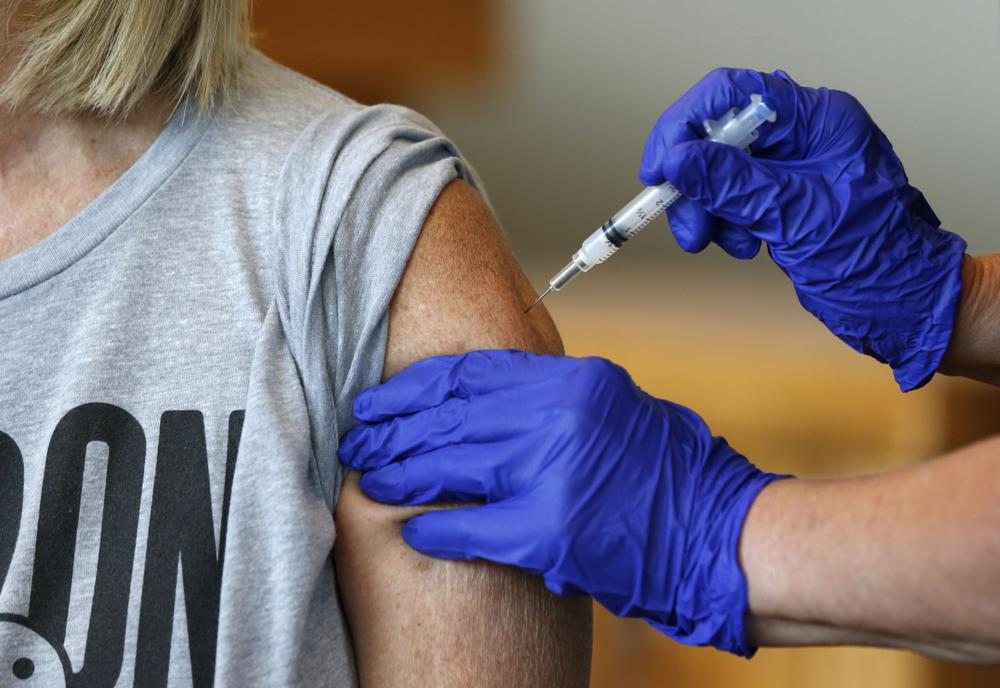
The COVID-19 curve in the U.S. is rising again after months of decline, with the number of new cases per day doubling over the past three weeks, driven by the fast-spreading delta variant, lagging vaccination rates, and Fourth of July gatherings. Confirmed infections climbed to an average of about 23,600 a day on Monday, up from 11,300 on June 23, according to Johns Hopkins University data. And all but two states — Maine and South Dakota — reported that case numbers have gone up over the past two weeks. “It is certainly no coincidence that we are looking at exactly the time that we would expect cases to be occurring after the July Fourth weekend,” said Dr. Bill Powderly, co-director of the infectious-disease division at Washington University’s School of Medicine in St. Louis. At the same time, parts of the country are running up against deep vaccine resistance, while the highly contagious mutant version of the coronavirus that was first detected in India is accounting for an ever-larger share of infection. Nationally, 55.6% of all Americans have received at least one COVID-19 shot, according to the Centers for Disease Control and Prevention. The five states with the biggest two-week jump in cases per capita all had lower vaccination rates: Missouri, 45.9%; Arkansas, 43%; Nevada, 50.9%; Louisiana, 39.2%; and Utah, 49.5%. Even with the latest surge, cases in the U.S. are nowhere near their peak of a quarter-million per day in January. And deaths are running at under 260 per day on average after topping out at more than 3,400 over the winter — a testament to how effectively the vaccine can prevent serious illness and death in those who happen to become infected. Still, amid the rise, health authorities in places such as Los Angeles County and St. Louis are begging even immunized people to resume wearing masks in public. And Chicago officials announced Tuesday that unvaccinated travelers from Missouri and Arkansas must either quarantine for 10 days or have a negative COVID-19 test. Meanwhile, the Health Department in Mississippi, which ranks dead last nationally for vaccinations, began blocking posts about COVID-19 on its Facebook page because of a “rise of misinformation” about the virus and the vaccine. Mississippi officials are also recommending that people 65 and older and those with chronic underlying conditions stay away from large indoor gatherings because of a 150% rise in hospitalizations over the past three weeks. In Louisiana, which also has one of the nation’s lowest vaccination rates, officials in the city of New Orleans said Tuesday that they are likely to extend until fall virus-mitigation efforts currently in place at large sporting and entertainment gatherings, including mask mandates or requirements that attendees be vaccinated or have a negative COVID-19 test. State health officials said cases of the coronavirus are surging, largely among nonvaccinated people. But the political will may not be there in many states fatigued by months of restrictions. In Michigan, Democratic Gov. Gretchen Whitmer is facing a drive to repeal a law that she used to set major restrictions during the early stages of the pandemic. And Republican Gov. Kay Ivey of Alabama pushed back against the idea that the state might need to reimpose preventive measures as vaccinations lag and hospitalizations rise. “Alabama is OPEN for business. Vaccines are readily available, and I encourage folks to get one. The state of emergency and health orders have expired. We are moving forward,” she said on social media. Dr. James Lawler, a leader of the Global Center for Health Security at the University of Nebraska Medical Center in Omaha, said bringing back masks and limiting gatherings would help. But he acknowledged that most of the places seeing higher rates of the virus “are exactly the areas of the country that don’t want to do any of these things.” Lawler warned that what is happening in Britain is a preview of what’s to come in the U.S. “The descriptions from regions of the world where the delta variant has taken hold and become the predominant virus are pictures of ICUs full of 30-year-olds. That’s what the critical care doctors describe, and that’s what’s coming to the U.S.,” he said. He added: “I think people have no clue what’s about to hit us.” President Joe Biden is putting a dose of star power behind the administration’s efforts to get young people vaccinated. Eighteen-year-old actress, singer, and songwriter Olivia Rodrigo will meet with Biden and Dr. Anthony Fauci on Wednesday. While the administration has had success vaccinating older Americans, young adults have shown less urgency to get the shots. Some, at least, are heeding the call in Missouri after weeks of begging, said Erik Frederick, chief administrative officer of Mercy Hospital Springfield. He tweeted that the number of people getting immunized at its vaccine clinic has jumped from 150 to 250 daily. “That gives me hope,” he said. Republished with the permission of the Associated Press.
Pfizer to discuss vaccine booster with U.S. officials Monday

Pfizer says it plans to meet with top U.S. health officials Monday to discuss the drugmaker’s request for federal authorization of a third dose of its COVID-19 vaccine as President Joe Biden’s chief medical adviser acknowledged that “it is entirely conceivable, maybe likely” that booster shots will be needed. The company said it was scheduled to have the meeting with the Food and Drug Administration and other officials Monday, days after Pfizer asserted that booster shots would be needed within 12 months. Pfizer’s Dr. Mikael Dolsten told The Associated Press last week that early data from the company’s booster study suggests people’s antibody levels jump five- to 10-fold after a third dose, compared to their second dose months earlier — evidence it believes supports the need for a booster. On Sunday, Dr. Anthony Fauci didn’t rule out the possibility but said it was too soon for the government to recommend another shot. He said the Centers for Disease Control and Prevention and the FDA did the right thing last week by pushing back against Pfizer’s assertion with their statement that they did not view booster shots as necessary “at this time.” Fauci said clinical studies and laboratory data have yet to fully bear out the need for a booster to the current two-shot Pfizer and Moderna vaccines or the one-shot Johnson & Johnson regimen. “Right now, given the data and the information we have, we do not need to give people a third shot,” he said. “That doesn’t mean we stop there. … There are studies being done now ongoing as we speak about looking at the feasibility about if and when we should be boosting people.” He said it was quite possible in the coming months “as data evolves” that the government may urge a booster based on such factors as age and underlying medical conditions. “Certainly it is entirely conceivable, maybe likely at some time, we will need a boost,″ Fauci said. Monday’s planned meeting between Pfizer and U.S. health officials was first reported by The Washington Post. Currently, only about 48% of the U.S. population is fully vaccinated. Some parts of the country have far lower immunization rates, and in those places, the delta variant is surging. Last week, Dr. Rochelle Walensky, the CDC director, said that’s leading to “two truths” — highly immunized swaths of America are getting back to normal while hospitalizations are rising in other places. Fauci said it was inexplicable that some Americans are so resistant to getting a vaccine when scientific data show how effective it is in staving off COVID-19 infections and hospitalizations, and he was dismayed by efforts to block making vaccinations more accessible, such as Biden’s suggestion of door-to-door outreach. Gov. Asa Hutchinson, R-Ark., agreed Sunday that there is vaccine resistance in Southern and rural states like his because “you have that more conservative approach, skepticism about government.” Describing his efforts to boost vaccinations in his state, which is seeing rising infections, Hutchinson said, “no one wants an agent knocking on a door,” but “we do want those that do not have access otherwise to make sure they know about it.” The grassroots component of the federal vaccination campaign has been in operation since April when supplies of shots began outpacing demand. It was outlined and funded by Congress in the $1.9 trillion COVID-19 relief bill passed in March and overwhelmingly is carried out by local officials and private-sector workers, and volunteers. Rep. Adam Kinzinger, R-Ill., blasted opposition to vaccination efforts from some GOP lawmakers as “absolute insanity.” He said House Republican leader Kevin McCarthy of California and others in the party need to speak out against “these absolute clown politicians playing on your vaccine fears for their own selfish gain.” Fauci appeared on CNN’s “State of the Union,” ABC’s “This Week,” and CBS’ “Face the Nation”; Hutchinson spoke on ABC, and Kinzinger was on CNN. Republished with the permission of the Associated Press.
Mo Brooks calls for Anthony Fauci’s termination in midst of controversial email release

Dr. Anthony Fauci, arguably America’s most well-recognized health official in the battle against COVID-19, assumed a central role in political controversy following a newly released slough of last year’s emails, giving rise to concerns about COVID-19’s origin and the controversial scientific research U.S taxpayers have funded. Yesterday, Congressman Mo Brooks (AL-05) joined several of his colleagues in a news conference to discuss auditing the correspondence and financial statements of Dr. Fauci. Fauci is no stranger to the spotlight, as the immunologist was one of the world’s most frequently-cited scientists across all scientific journals from 1983 to 2002, in addition to the world’s 10th most-cited HIV/AIDS researcher from 1996-2006. He has advised seven Presidents and was awarded a Presidential Medal of Freedom by President George H.W. Bush in 2008 for his efforts on an AIDS relief program. Serving as director of the National Institute of Allergy and Infectious Diseases (NIAID) since 1984, Fauci is perhaps more widely recognized for leading the nation’s COVID-19 response as a White House coronavirus advisor during the Trump Administration. He continues to lead the nation’s pandemic response during his current role as chief medical advisor in the Biden Administration. However, Fauci’s consistently shifting narratives throughout the pandemic, in addition to frequent opposition towards President Donald Trump’s leadership, resulted in a sizable number of public critics, many of which included Trump White House officials. Peter Navarro, a Harvard-trained economist and China hawk who served as a top trade and economic policy advisor to President Trump, publicly criticized Dr. Fauci in a USA Today op-ed, outlining the many instances Fauci was mistaken during the pandemic. USA Today promptly attached a remorseful precursor to the article; an apologetic note addressed to readers for publishing any criticism of Fauci. One of the most notable examples Navarro specifies is the predictive memos he sent in January and February 2020, which grimly anticipated COVID-19 to be a deadly and impactful global pandemic. Senior officials shrugged off these warnings, including Fauci, due to Navarro’s hawkish views on China. “The lack of immune protection or an existing cure or vaccine would leave Americans defenseless in the case of a full-blown coronavirus outbreak on US soil,” Navarro’s January 29 memo to the National Security Council states. “The lack of protection elevates the risk of the coronavirus evolving into a full-blown pandemic, imperiling the lives of millions of Americans.” Weeks after Navarro’s warning was sent out, Fauci assured the media just how worried the American people should be about the pandemic when he expressed, “The danger of getting coronavirus now is just minusculely low,” Fauci stated. “As of today, on the 17th of February, the risk is really relatively low.” Since then, public criticism of Fauci continues to escalate as 3,000 pages of his emails from March and April 2020 were obtained under the Freedom of Information Act (FOIA) following a lawsuit filed by taxpayer watchdog group, the White Coast Waste Project. “Taxpayers have a right to know what the NIH knew about how its money was being spent at the Wuhan animal lab, and what NIH knew about a potential lab leak in late 2019 and early 2020,” stated Justin Goodman, vice president of advocacy and public policy at the White Coat Waste Project. “Transparency and accountability at home and abroad are critical in the quest to identify the origin of the COVID-19 pandemic in order to prevent another outbreak.” Fauci’s obtained emails point to the fact that he was indeed warned of the possibility that COVID-19 was engineered, a theory he remained adamantly opposed to throughout the pandemic. Kristian Andersen, the head of a viral genomics lab at Scripps Research in La Jolla, CA, emailed Fauci in February 2020 entertaining the possibility of COVID-19’s lab-based origin, “The unusual features of the virus make up a really small part of the genome (<0.1%) so one has to look really closely at all the sequences to see that some of the features (potentially) look engineered.” This week, Anderson addressed his involvement in these recently released emails, assuring that his newfound research discourages any lab-based scenarios while also claiming it is scientifically impossible to determine the origins of the pandemic, “As we stated in our article last March, it is currently impossible to prove or disprove specific hypotheses of SARS-CoV-2 origin.” Additionally, these emails raise questions surrounding the type of research U.S. taxpayers are funding. Under Fauci’s four-decade-long leadership, the NAIAD resides within the National Institute of Health (NIH), which allocates 80% of its federal funds to scientific research, including grants to foreign organizations. Fauci swore under oath that no taxpayer funds were used to fund research in Wuhan. However, in a later congressional hearing, he stated that the NIH earmarked $600,000 to study coronaviruses in Wuhan. NIH Director Dr. Francis Collins confirmed that $3.7 million in federal funds were sent to EcoHealth Alliance, a global nonprofit, of which $600,000 went to the Wuhan Institute of Virology (WIV). Fauci’s emails show a message received from the President of EcoHealth Alliance, Peter Daszak, thanking him for rejecting any lab-leak theories in April 2020. Daszak wrote to Fauci, “I just wanted to say a personal thank you on behalf of our staff and collaborators, for publicly standing up and stating that the scientific evidence supports a natural origin for COVID-19 from a bat-to-human spillover, not a lab release from the Wuhan Institute of Virology.” Daszak adds, “Your comments are brave, and coming from your trusted voice, will help dispel the myths being spun around the virus’ origins.” These concerns have led Congressional Republicans, including House Minority Leader Kevin McCarthy (R-CA), to call for Fauci’s dismissal from his role as NIAID director. Yesterday, Congressman Mo Brooks joined GOP lawmakers in a press conference to announce his support of the Fire Fauci Act. The bill would bring Dr. Fauci’s taxpayer salary to $0 and will require the Senate to confirm another individual to fill his position. “Dr. Fauci is consistent in just one thing and that is inconsistency,” Brooks said. “Why
Big gaps in vaccine rates across the US worry health experts

A steady crowd of people flowed into the New England Patriots’ stadium for their second dose of the COVID-19 vaccine this week in Massachusetts, which is nearing its goal of vaccinating more than 4 million and plans to close its biggest clinics in little more than a month. In the Deep South, meanwhile, one of the largest clinics in Alabama shut down Wednesday, and others will follow in the coming weeks because demand for the shot has plunged. “They didn’t have long enough to test it,” said James Martin, 68, explaining why he has no plans to get the vaccine as he stopped for cigarettes at a convenience store in Clanton, Alabama. “They don’t know what the long-term effect is. That’s what makes me skeptical.” A month after every adult in the U.S. became eligible for the vaccine, a distinct geographic pattern has emerged: The highest vaccination rates are concentrated in the Northeast, while the lowest ones are mostly in the South. Experts say the gap reflects a multitude of factors, including political leanings, religious beliefs, and education and income levels. Close to 160 million Americans — 48% of the population — have received at least one dose of a COVID-19 vaccine, and 125 million are fully vaccinated against the virus. New England and Northeastern states account for eight of the top 10 in vaccination rates, with Vermont No. 1 as of last Friday, according to the Centers for Disease Control and Prevention. Nearly 64% of its population has received as least one dose. Following right behind are Massachusetts, Hawaii, New Hampshire, Maine, Connecticut, Rhode Island, New Jersey, Pennsylvania, and New Mexico, all of them at 54% or higher. Eight Southern states are in the bottom 10, all of which are under 40%. Mississippi was dead last at 32%, followed by Louisiana, Alabama, Wyoming, Idaho, Tennessee, Arkansas, Georgia, West Virginia, and South Carolina. Closing the gaps is vital to controlling the virus that has killed 588,000 people in the U.S., health experts say. The vaccination drive has helped drive U.S. cases down to their lowest level since last June, at around 30,000 a day on average, and reduced deaths to about 570 a day, a level not seen since last July. “Low vaccination rates will leave room for the virus to circulate, re-emerge and possibly form new variants,” said Tara Kirk Sell, a senior scholar at the Johns Hopkins Center for Health Security. “High vaccination rates are critical to keeping the disease under control, especially when we get back to the fall and winter.” The divides aren’t just limited to states — there are marked differences between urban and rural places, from county to county and from one neighborhood to another. The disparities are even more glaring when looking at individual places around the U.S.: Vermont has four counties where 75% of the residents have had at least one dose, while there are 11 Mississippi counties with under 25% vaccinated. Roddy Carroll has seen both sides from where he works in technology sales in Atlanta and where he grew up in northern Georgia. “There’s a pretty stark difference,” he said. Back home in rural Murray County, only 1 in 4 residents have rolled up their sleeves for a shot. Carroll blames conservative politicians for sowing doubts that have made people reluctant. “They’re more willing to listen to conspiracy theorists than doctors who know how vaccines work,” he said. “You’re talking about people you’ve known all their life. But you hear them say those things, and you think, `How well did I know them?’” Those anti-vaccine beliefs have led to some uncomfortable conversations with his family, Carroll said. “I don’t know anybody who hasn’t had tense moments like that,” he said. Dr. Eric Topol, head of the Scripps Research Translational Institute, said the gaps in COVID-19 vaccination can be traced directly to political influences, particularly what he called “anti-science” attitudes among Republican leaders, who were skeptical about the value of masks, too. Getting more people vaccinated will take continued education, incentives, and “head-on” confrontation of misinformation, Topol said. He expects U.S. regulators to grant full approval to the vaccines soon, which will give employers, the military, and health systems the green light to require vaccination. “That will make the biggest difference,” Topol said. Gail Borel, of Plymouth, Massachusetts, a nurse who arrived with her husband at Gillette Stadium on Wednesday to get their second doses, said she was initially reluctant. But she said she decided to go through with it after her employer said she could be held liable if she refused the vaccine and got patients sick. Her husband, Tom, didn’t share her concern. “Everybody I know just wants to get it over with. If this is the path to get it over with, then this is what we’re willing to do,” he said. “I just want this to be over. It’s how we stop wearing masks and how people stop getting sick.” In Massachusetts, where 62% have had at least one dose, there has been little resistance to public health orders during the pandemic, and state leaders have kept tight restrictions on gatherings and businesses, drawing praise from officials such Dr. Anthony Fauci, the nation’s top infectious disease expert. Some credit the success to the state’s deep ties to education and health care. The Boston area alone has dozens of universities, including Harvard and MIT, and scores of biotechnology companies, including the vaccine maker Moderna. Nationwide, rural counties are behind urban places in their COVID-19 vaccination efforts — 39% of adults in rural counties had received at least one shot compared with 46% in urban counties as of April 10, according to an analysis released Tuesday by the CDC. The rural-urban gap exists among women, men, and both younger and older adults, the CDC said. In Alabama’s Chilton County, a peach-farming area, the longtime mayor of Clanton died of the virus last year along with 85 others in the county. Yet less than 17% of its population is fully vaccinated, giving it one of the


