Alabama to expand COVID-19 vaccine eligibility on March 22

Alabama is expanding eligibility later this month for COVID-19 vaccinations to more frontline workers, residents with certain chronic health conditions, and people 55 and older, state officials announced Friday. “We have been concerned that many people at high risk and others engaged in close-contact work have not been eligible to receive the vaccine yet, but with the additional vaccine supply we are better able to meet the needs of Alabama residents,” Gov. Kay Ivey said in a statement. The expansion, starting March 22, will add over 2 million people to the groups who can receive a COVID-19 vaccination in Alabama, roughly doubling the number of people now eligible. The dramatic increase comes at a time when demand continues to exceed supply and will increase the competition to find shots. State Health Officer Scott Harris said Alabama expanded eligibility because of the expectations of the public — particularly as they see people in other states getting shots — and health officials’ expectations that the supply will jump over the coming weeks. “I would just encourage people to please remember to be patient. They have been patient for so long and we are really very very close to having enough vaccine to go around. I think in a month, probably six weeks at least, there is going to be more than an adequate supply of vaccine,” Harris told reporters Friday. The new eligible groups include more frontline workers; people 55 and older; those with intellectual and developmental disabilities; and residents age 16 to 64 with certain high-risk medical conditions. The qualifying medical conditions include cancer, chronic kidney disease, diabetes, smoking, obesity, sickle cell disease and heart conditions. More workers will also be eligible for the shots, including restaurant staff, transportation workers, construction workers, bank tellers, legal professionals and members of the news media. Alabama currently ranks near the bottom for the percentage of the population that has been vaccinated, according to numbers from the Centers for Disease Control and Prevention. About 16.1% of the state’s 4.9 million people have received at least one vaccination dose. Harris said the department conducted surveys to try to gauge vaccine hesitancy and found up to 30% of adults are reluctant to take the vaccine. Some of those are “people who simply just need better information. We need to find away to educate them,” Harris said. He said others are people who have “other ideas about vaccinations in general or even about the coronavirus event in general.” Since the pandemic began, more than 500,000 Alabamians have tested positive for COVID-19. Ivey has directed flags to be placed at half-staff on Saturday to honor and remember the more than 10,000 Alabamians who lost their lives to the COVID-19 pandemic, her office announced. The announcement of the expanded eligibility comes a year after the first COVID-19 case was identified in the state. Harris noted the remarkable work that went onto developing the vaccines. “The vaccine response to COVID-19 is really going to be like landing on the moon was for some of us of a certain age,” Harris said. Republished with the permission of the Associated Press.
Joe Biden vows enough vaccine for all US adults by end of May

President Joe Biden said Tuesday the U.S. expects to take delivery of enough coronavirus vaccine for all adults by the end of May — two months earlier than anticipated — and he pushed states to get at least one shot into the arms of teachers by the end of May to hasten school reopenings. Biden also announced that drugmaker Merck will help produce rival Johnson & Johnson’s newly approved one-shot vaccine, likening the partnership between the two drug companies to the spirit of national cooperation during World War II. “We’re now on track to have enough vaccine supply for every adult in America by the end of May,” Biden said. Despite the stepped-up pace of vaccine production, the work of inoculating Americans could extend well into the summer, officials said, depending both on the government’s capacity to deliver doses and Americans’ willingness to roll up their sleeves. Biden’s announcements quickly raised expectations for when the nation could safely emerge from the pandemic with the promise of speedier vaccinations, but even as he expressed optimism, Biden quickly tempered the outlook for a return to life as it was before the virus hit. “I’ve been cautioned not to give an answer to that because we don’t know for sure,” Biden said, before saying his hope for a return to normal was sometime before “this time next year.” As Biden spoke, states across the country were moving to relax virus-related restrictions. This despite the objections of the White House and the nation’s top infectious disease expert, Dr. Anthony Fauci, who have warned against any relaxation of virus protocols until more Americans are vaccinated. In Texas, GOP Gov. Greg Abbott moved to lift his state’s mask-wearing mandate and a host of other limitations. Michigan’s Democratic Gov. Gretchen Whitmer eased capacity limits on restaurants and both public and residential gatherings. Fauci has previously said the nation must achieve a vaccination rate of about 80% to reach “herd immunity.” Only about 8% of the population has been fully vaccinated, according to the Centers for Disease Control and Prevention, though the pace of vaccination has been increasing. The U.S. set a new daily record for injections last Thursday and Friday. In hopes of increasing vaccinations even further. the Biden administration told governors to make preparations to administer even more doses in the coming weeks. More shots are also headed toward the federally backed program to administer doses in retail pharmacies, which federal officials believe can double or triple their pace of vaccination. More than 800,000 doses of the J&J vaccine will also be distributed this week to pharmacies, on top of the 2.4 million they are now getting from Pfizer and Moderna. Those pharmacies will be key in getting the vaccines into the arms of teachers — particularly in the roughly 20 states where they have not been prioritized for shots. The aim is to help reopen schools to better educate students who have been at risk of falling behind during the pandemic and reduce the burden on parents who have had to choose between childcare and a job. “Let’s treat in-person learning as the essential service that it is,” Biden said. Teachers will be able to sign up directly through participating retail pharmacies, the administration said. White House press secretary Jen Psaki also announced Tuesday that the federal government was increasing supply of the Moderna and Pfizer vaccines to states next week to 15.2 million doses per week, up from 14.5 million previously. States will also receive 2.8 million doses of the J&J shot this week. On a call with governors Tuesday, White House coronavirus coordinator Jeff Zients said states should prepare to administer 16 million to 17 million total weekly doses of Pfizer and Moderna vaccines by the end of March, climbing to 17 million to 18 million weekly by early April. The supply of J&J doses to states, expected to dip after the initial shipment this week, will climb to 4 million to 6 million weekly doses by the end of March and 5 million to 6 million doses weekly through the end of April. Officials have said J&J faced unexpected production issues with its vaccine and produced only 3.9 million doses before being cleared for emergency use authorization on Saturday. The company has promised to deliver 100 million doses by the end of June. Before the approval of the J&J shot, Biden had suggested that it would take until the end of July to have enough vaccine for every adult in the U.S. Facing questions about the company’s slipping delivery schedule, J&J Vice President Richard Nettles told lawmakers on Capitol Hill last week that the company had faced “significant challenges” because of its “highly complex” manufacturing process. Psaki said that an “across-the-administration effort” was required to get the two historic rivals to work together on the vaccines, even though conversations between J&J and Merck have been going on for months. “There’s a difference between conversations and it moving forward,” she said. The White House said Merck would devote two plants to the production process. One would make the vaccine and the other would handle inserting the vaccine into vials and ensuring strict quality controls. Psaki said the Biden administration was using its powers under the Defense Production Act to help Merck retool to work on the production. Still, it was not immediately clear when the effect of Merck’s assistance would be reflected in supply. Federal officials have cautioned that setting up the highly specialized manufacturing lines to produce vaccines would take months. Compared to the two-dose versions produced by Moderna and Pfizer, the J&J vaccine is less resource-intensive to distribute and administer, making it critical for U.S. plans to spread vaccinations around the world — but only once Americans are inoculated. The J&J vaccine can be stored for months at refrigerated temperatures, rather than frozen, and doesn’t require patients to return for a second dose three or four weeks later. J&J has set up a global production network that includes brewing bulk
States easing virus restrictions despite experts’ warnings

With the U.S. vaccination drive picking up speed and a third formula on the way, states eager to reopen for business are easing coronavirus restrictions despite warnings from health experts that the outbreak is far from over and that moving too quickly could prolong the misery. Massachusetts on Monday made it much easier to grab dinner and a show. In Missouri, where individual communities get to make the rules, the two biggest metropolitan areas — St. Louis and Kansas City — are relaxing some measures. Iowa’s governor recently lifted mask requirements and limits on the number of people allowed in bars and restaurants, while the town of Lawrence, home to the University of Kansas, now lets establishments stay open until midnight. Mike Lee, who owns Trezo Mare Restaurant & Lounge in Kansas City, said he hopes increased vaccine access, combined with warmer weather, will improve business. “I think that people are excited to put this past them and be able to start to get back to their ways of doing things,” Lee said. The push to reopen comes as COVID-19 vaccine shipments to the states are ramping up. Nearly 20% of the nation’s adults — or over 50 million people — have received at least one dose of vaccine, and 10% have been fully inoculated 2 1/2 months into the campaign to snuff out the virus, according to the Centers for Disease Control and Prevention. Johnson & Johnson shipped out nearly 4 million doses of its newly authorized, one-shot COVID-19 vaccine Sunday night to be delivered to states for use starting on Tuesday. The company will deliver about 16 million more doses by the end of March and a total of 100 million by the end of June. That adds to the supply being distributed by Pfizer and Moderna and should help the nation amass enough doses by midsummer to vaccinate all adults. The White House is encouraging Americans to take the first dose available to them, regardless of manufacturer. In New York City, where limited indoor dining has resumed, officials said the J&J vaccine will help the city to inoculate millions more people by summer, including through door-to-door vaccinations of homebound senior citizens. But the efforts come with strong warnings from health officials against reopening too quickly, as worrisome coronavirus variants spread. On Monday, the head of the CDC, Dr. Rochelle Walensky, urgently warned state officials and ordinary Americans not to let down their guard, saying she is “really worried about reports that more states are rolling back the exact public health measures that we have recommended.” “I remain deeply concerned about a potential shift in the trajectory of the pandemic,” she said. “We stand to completely lose the hard-earned ground that we have gained.” Cases and hospitalizations have plunged since the end of January, and deaths have also dropped sharply, but they are still running at dangerously high levels and have even risen slightly over the past several days. “We cannot be resigned to 70,000 cases a day and 2,000 daily deaths,” Walensky said. Overall, the outbreak has killed more than a half-million Americans. The vaccine already is contributing to a decrease in severe cases and deaths among older people, and is “quickly becoming a bigger contributor” nationally, Justin Lessler, an expert in infectious diseases at Johns Hopkins University, said in an email. “I suspect we will see it overtake natural infection as the biggest driver of immunity late spring earliest, more likely midsummer,” Lessler said. Dr. Amesh Adalja, an infectious disease specialist at Johns Hopkins University, said he believes states and cities have leeway to ease some restrictions because hospitals no longer are at capacity in most communities. But “I do think that masks are likely going to need to be kept in place for some time until we get more of our vulnerable populations vaccinated,” he said. “It is important for restaurants who are increasing their capacity to remember that we are still in a pandemic and to continue to follow some of those rules,” Adalja said. The Biden administration wants to see all three vaccines distributed evenly, while also acknowledging that the easy-to-handle J&J vaccine will be used in pop-up mobile sites and locations without freezer storage capacity. States are hoping that the surging vaccine supply will help tamp down new infections. In Massachusetts, Gov. Charlie Baker lifted restaurant capacity limits entirely. Theaters can open at 50% capacity, with a maximum of 500 people. And capacity limits across all businesses have been raised to 50%. Las Vegas on Monday became the latest of the nation’s largest school districts to return children to classrooms. Pre-K children to third graders will go back two days a week, with other grades to be phased in by early April. And in California, Gov. Gavin Newsom and legislative leaders reached an agreement aimed at getting most children back in classrooms by the end of March. Under the deal announced Monday, school districts could receive up to $6.6 billion if they reopen by March 31. The U.S. ranks fourth in the world, behind Israel, the United Arab Emirates, and Britain, in the number of doses administered relative to the population, according to data compiled by the University of Oxford. President Joe Biden fell well short of his goal of setting up 100 new federally operated mass-vaccination sites by the end of February, with just seven up and running. White House vaccination coordinator Jeff Zients also acknowledged that scheduling of vaccination appointments “remains too difficult in too many places.” But he said the White House is working with states to improve scheduling systems and is exploring federal support for call centers to make it easier for people to get appointments. Republished with the permission of the Associated Press.
Beyond 100M: Joe Biden team aiming for bigger vaccine numbers

It sounded so ambitious at first blush: 100 million vaccination shots in 100 days. Now, one month into his presidency, Joe Biden is on a glide path to attain that goal and pitching well beyond it to the far more ambitious and daunting mission of vaccinating all eligible adults against the coronavirus by the end of the summer. Limited supply of the two approved COVID-19 vaccines has hampered the pace of vaccinations — and that was before extreme winter weather delayed the delivery of about 6 million doses this past week. But the United States is on the verge of a supply breakthrough as manufacturing ramps up and with the expectation of a third vaccine becoming available in the coming weeks. That means the act of delivering injections will soon be the dominant constraint, and it’s prompting the Biden administration to push to dramatically expand the universe of those who will deliver injections and where Americans will meet them to get their shots. “It’s one thing to have the vaccine, and it’s very different to get it in someone’s arms,” Biden said Friday as he toured Pfizer’s manufacturing plant in Portage, Michigan. The company is set to double its pace of vaccine deliveries in the coming weeks. Since their approval in December, more than 75 million doses of the two-shot-regimen Moderna and Pfizer vaccines have been distributed, of which 63 million have been injected, reaching 13% of Americans. Nearly 45 million of those doses have been administered since Biden’s inauguration on Jan. 20. The pace of deliveries of those vaccines is about to take off. About 145 million doses are set for delivery in the next 5 1/2 weeks, with an additional 200 million expected by the end of May and a further 200 million by the end of July. That’s before the anticipated approval by the Food and Drug Administration for emergency use of a third vaccine, from Johnson & Johnson. The single-dose J&J vaccine is expected to help speed the path to immunity and requires half the vaccination resources of the two-shot regimens. But there is no massive stockpile of J&J doses ready to roll out on Day One. “We’re going to be starting with only a few million in inventory,” White House COVID-19 coordinator Jeff Zients said this past week. Still, when combined with the anticipated increases in the other vaccines, the J&J doses could prove the pivotal advance in delivering enough shots for nearly all American adults by the end of June, at least a month earlier than currently anticipated. The daily inoculation average climbed to 1.7 million shots per day last week, but as many as double that number of doses are soon expected to be available on average each day. The focus of Biden’s team is now quickly shifting to ensuring those doses can get used, though the administration has resisted the calls of some health experts to publicly set a “moonshot” target for how many daily doses it hopes to deliver. Biden first set his target of 100 million doses in 100 days on Dec. 8, days before the first vaccines received emergency use authorization. By Inauguration Day, it was clear the U.S. was on course to attain that goal. Dr. Leana Wen, an emergency physician and public health professor at George Washington University, said she would like to see the administration commit to a more ambitious 3 million shot-per-day target. “I want to see them put that stake in the ground and ask everyone to help them achieve that goal,” she said. The current pace of vaccination dipped markedly in recent days as winter weather shuttered administration sites in Texas and across the South, and icy conditions stranded supplies at shipping hubs in Louisville, Kentucky and Memphis, Tennessee. One-third of the delayed doses have already been delivered, Dr. Anthony Fauci, the nation’s top infectious disease specialist, announced Sunday. The White House anticipates that remaining delayed doses will be injected by March 1 and that the daily pace of vaccinations will continue to climb. Much of the increase, according to data from the Centers for Disease Control and Prevention, comes from people receiving their second dose of the Moderna or Pfizer vaccine. The pace of first-dose vaccinations, meanwhile, has been largely steady over the past several weeks, hovering around an average of 900,000 shots per day. Increasing both the rate of first-dose administrations and the rate of overall vaccinations will be key to achieving herd immunity — estimated to require vaccination of about 80% of the population — in hopes of ending the pandemic and curtailing the emergence of potentially even more dangerous “mutant” strains of the coronavirus. That means keeping demand high. The administration has expressed concerns about public surveys showing that tens of millions of Americans are reluctant to get the vaccine and it is stepping up public outreach to overcome that hesitancy as the U.S. death toll nears 500,000 — “a terribly historic milestone in the history of this country,” as Fauci put it, and “we’re still not out of it.” Dr. Cyrus Shahpar, the White House COVID-19 data director, said in an interview that the administration is “focused on going out to communities and making sure people know these vaccines are safe and how they can get them, with a goal of vaccinating nearly all Americans,.” The administration has also turned its focus toward identifying new delivery paths for the vaccines beyond those already used by states, including federally-run mass vaccination sites, smaller community health centers and retail pharmacies. The White House’s goal is to stand up the sites now so that they will be ready to handle the influx of vaccine in the coming weeks. “They can push a lot more volume through those channels, through those big box stores, through the community health centers,” Scott Gottlieb, a former Trump administration FDA commissioner, told MSNBC on Friday. He praised the Biden administration for setting up those sites in advance. The Pentagon, at the request of the Federal Emergency Management Agency, has started deploying thousands of active-duty troops to open mass vaccination centers
Joe Biden says U.S. is securing 600 million vaccine doses by July

President Joe Biden said Thursday that the U.S. will have enough supply of the COVID-19 vaccine by the end of the summer to inoculate 300 million Americans. Biden made the announcement at the sprawling National Institutes of Health complex just outside Washington as he visited some of the nation’s leading scientists on the frontlines of the fight against the disease. He toured the Viral Pathogenesis Laboratory that created the COVID-19 vaccine now manufactured by Moderna and being rolled out in the U.S. and other countries. The U.S. is on pace to exceed Biden’s goal of administering 100 million vaccine doses in his first 100 days in office, with more than 26 million shots delivered in his first three weeks. “That’s just the floor,” Biden said. “Our end goal is beating COVID-19.” Biden announced on Thursday that the U.S. had secured contractual commitments from Moderna and Pfizer to deliver the 600 million doses of vaccine by the end of July — more than a month earlier than initially anticipated. “We’re now on track to have enough supply for 300 million Americans by the end of July,” he announced. The pace of injections could increase further if a third coronavirus vaccine from drugmaker Johnson & Johnson receives approval from the Food and Drug Administration. Speaking with Dr. Anthony Fauci, the nation’s top infectious-disease specialist, Biden emphasized that his administration is doing everything possible to increase the vaccine supply and the country’s capacity to deliver injections into arms. “It’s been a hell of a learning process,” Biden said. Biden, wearing a mask, used his remarks to criticize President Donald Trump, saying he inherited “no plan to vaccinate most of the country.” “It is no secret that the vaccination program was in much worse shape than my team and I anticipated,” he said. To date, the Biden administration has deployed active-duty troops to help stand up mass vaccination sites in several states, as it looks to lay the groundwork for increasing the rate of vaccinations once more supply is available. The Viral Pathogenesis Laboratory is led by Dr. Barney Graham, whose team made critical discoveries years ago that laid the groundwork for rapid development of that and other COVID-19 vaccines. Before the pandemic erupted, one of Graham’s research fellows, Dr. Kizzmekia Corbett, had been using those earlier findings to develop a vaccine for MERS, a cousin of COVID-19. On the tour, Biden was shown the lab bench where researchers sequenced the virus and developed the precursor of the Moderna vaccine. Armed with their prior research, Corbett and Graham had a head start when Chinese scientists shared the genetic map of the new coronavirus in January 2020. They already knew how to make spike proteins, which coat the surface of the new coronavirus and its MERS relative, that were stable enough to be used as a key vaccine ingredient. Within days, the NIH had sent instructions to Moderna to brew up doses, and Corbett and her colleagues were setting up the key lab and animal tests that would eventually prove they were on the right track. Republished with the permission of the Associated Press.
Virus vaccine available at dozens of Alabama Walmart stores
Vaccines against COVID-19 will soon be available at more than 70 Walmart and Sam’s Club stores across Alabama, the company and the state announced. The Arkansas-based retailer said people who meet the state’s eligibility requirements can begin signing up for appointments, and the immunizations begin Friday. Part of the program is meant to get the vaccine into areas without adequate medical services, the company said. That includes the south Alabama town of Brewton, which the company said was chosen to get the vaccine because other immunization sites are so far away. More than 1,000 Walmart and Sam’s Club pharmacies in Alabama and 21 other states are receiving federal vaccine allocations this week. Gov. Kay Ivey said the state was grateful for the doses but urged patience since each store will have a limited supply of vaccine. The state on Monday expanded vaccine eligibility to include everyone 65 and older; school workers; grocery store employees; some manufacturers; public transit workers; agriculture employees; state legislators and constitutional officers. As many as 1.5 million people now qualify for shots, up from about 700,000 previously. Republished with the permission of the Associated Press.
Alabama begins expanded COVID-19 vaccinations on Monday

Alabama on Monday will expand who is eligible to receive immunizations against COVID-19 but health officials cautioned there’s still not enough vaccine for everyone who qualifies for a shot. Beginning Monday, everyone 65 and older; educators; grocery store workers; some manufacturing workers; public transit workers; agriculture employees; state legislators, and constitutional officers will be eligible to get vaccinations. Previously only health care workers, first responders, nursing home residents, and people 75 and older were eligible. “If you are eligible for a vaccine, then we will get you one if want to take it. But it is not going to happen immediately for everyone. There is still going to be a while before we have enough,” Dr. Scott Harris, the state health officer, told reporters Friday. Harris said an estimated 1.5 million people will be eligible for the vaccinations. The Alabama Department of Public Health site has a map of providers providing shots. The state is opening large, drive-thru clinics in eight cities: Anniston, Birmingham, Dothan, Huntsville, Mobile, Montgomery, Selma, and Tuscaloosa. Harris said it will require 3 million doses to give the 1.5 million people the two shots required for maximum protection. The state has so far received 923,750 doses and is receiving about 70,000 per week. So far, 436,962 doses have been given. Harris said the remaining doses have someone’s name on it through appointments and clinics for a first or second shot. The University of Alabama at Birmingham announced it is opening an a third clinic, this one at Parker High School, for vaccinations. People do not have to be UAB patients but must fall in the statewide eligibility group. Birmingham Mayor Randall Woodfin urged people in the city to get vaccinated. “We are not out of the woods yet. So I really want to encourage the citizens of Birmingham to take advantage of this site,” Woodfin said. More than 8,500 people in Alabama have died of COVID-19, including confirmed and probable cases. More than 472,000 have tested positive. While the virus causes only mild or moderate symptoms for most people, it can be deadly for the elderly and people with serious health problems including diabetes, cardiovascular disease. Republished with the permission of the Associated Press.
Study finds COVID-19 vaccine may reduce virus transmission
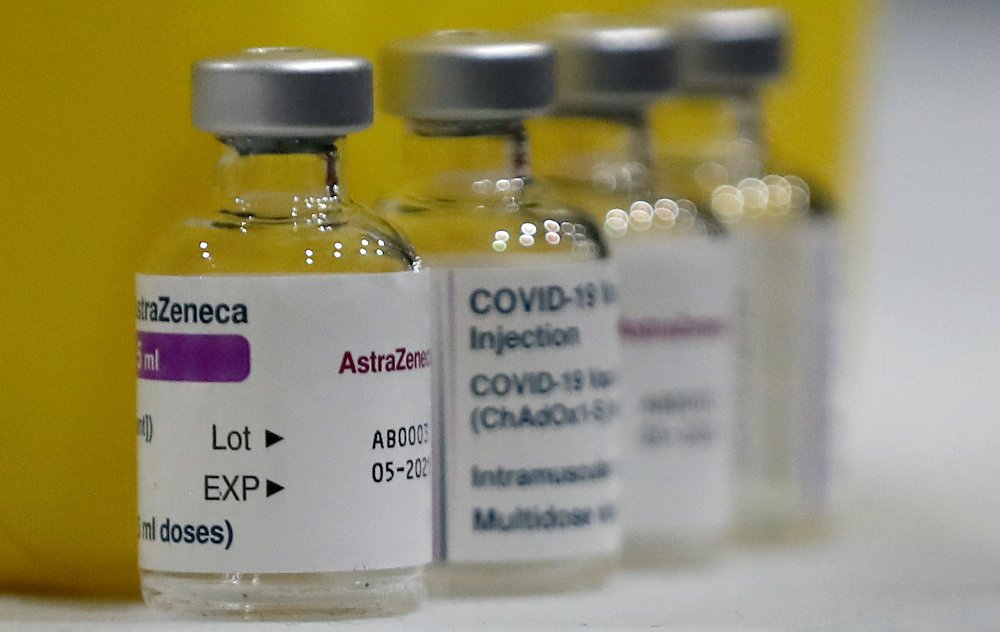
AstraZeneca’s COVID-19 vaccine shows a hint that it may reduce transmission of the virus and offers strong protection for three months on just a single dose, researchers said Wednesday in an encouraging turn in the campaign to suppress the outbreak. The preliminary findings from Oxford University, a co-developer of the vaccine, could vindicate the British government’s controversial strategy of delaying the second shot for up to 12 weeks so that more people can be quickly given a first dose. Up to now, the recommended time between doses has been four weeks. The research could also bring scientists closer to an answer to one of the big questions about the vaccination drive: Will the vaccines actually curb the spread of the coronavirus? It’s not clear what implications if any, the findings might have for the two other major vaccines being used in the West, Pfizer’s and Moderna’s. In the United States, Dr. Anthony Fauci, the nation’s top infectious disease expert, dismissed the idea of deliberately delaying second shots, saying the U.S. will “go by the science” and data from the clinical trials. The two doses of the Pifzer and Moderna vaccines are supposed to be given three and four weeks apart. Still, the research appears to be good news in the desperate effort to arrest the spread of the virus and also suggests a way to ease vaccine shortages and get shots into more arms more quickly. The makers of all three vaccines have said that their shots proved to be anywhere from 70% to 95% effective in clinical trials in protecting people from illness caused by the virus. But it was unclear whether the vaccines could also suppress transmission of the virus — that is, whether someone inoculated could still acquire the virus without getting sick and spread it to others. As a result, experts have been saying that even people who have been vaccinated should continue to wear masks and keep their distance from others. Volunteers in the British study underwent regular nasal swabs to check for the coronavirus, a proxy to try to answer the transmission question. The level of virus-positive swabs — combining volunteers who had asymptomatic infection with those who had symptoms — was 67% lower in the vaccinated group, the researchers reported. While not a direct measure, “that’s got to have a really beneficial effect on transmission,” Oxford lead researcher Sarah Gilbert told a meeting of the New York Academy of Sciences Wednesday. The researchers also looked at how likely people who have been vaccinated are to get a symptom-free infection. In one subset of volunteers, there were 16 asymptomatic infections among the vaccinated and 31 in an unvaccinated comparison group. Pfizer and Moderna also are studying the effect of their vaccines on asymptomatic infections. Only the Pfizer and Moderna vaccines are being used in the United States. Britain is using both AstraZeneca’s and Pfizer’s. AstraZeneca’s has also been authorized by the 27-nation European Union. Pfizer has not endorsed the British government’s decision to lengthen the time between doses. Mene Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said that no patients experienced severe COVID-19 or required hospitalization three weeks after receiving a first dose, and that effectiveness appeared to increase up to 12 weeks after the initial shot. “Our data suggest you want to be as close to the 12 weeks as you can” for the second dose, Pangalos said. British Health Secretary Matt Hancock said the study “backs the strategy that we’ve taken” to make sure more people have gotten at least one shot. Britain’s decision has been criticized as risky by other European countries. Stephen Evans of the London School of Hygiene and Tropical Medicine said the study’s suggestion that a single dose protected people for 12 weeks was “useful but not definitive.” He said that the authors themselves acknowledged their research was not designed to investigate the vaccine’s dosing schedule and that their conclusions were based on statistical modeling, not actual patients tracked over time. “It certainly isn’t very strong evidence, but there is also no indication this is the wrong thing to do,” Evans said of Britain’s strategy. One of the Oxford researchers, Dr. Andrew Pollard, said scientists also believe the AstraZeneca vaccine will continue to offer protection against new variants of COVID-19, though they are still waiting for data on that. Fast-spreading mutant versions have caused alarm around the world. “If we do need to update the vaccines, then it is actually a relatively straightforward process. It only takes a matter of months, rather than the huge efforts that everyone went through last year to get the very large-scale trials run,” Pollard told the BBC. Meanwhile, a U.N.-backed program to supply COVID-19 vaccines to the neediest people worldwide is gearing up after a troubled start. The COVAX Facility announced plans Wednesday for an initial distribution of some 100 million doses by the end of March and more than 200 million more by the end of June to dozens of countries. Nearly all of the doses expected for the first phase are due to come from AstraZeneca and its partner, the Serum Institute of India. The rollout will be contingent on the World Health Organization authorizing the AstraZeneca shot for emergency use, which is expected to happen this month. Some 190 countries and territories are participating in COVAX, which has seen rich nations scoop up vaccine supplies, sometimes at premium prices. The pandemic’s worldwide death toll has eclipsed 2.2 million, including about 447,000 in the U.S., according to Johns Hopkins University data. New cases per day in the U.S. and the number of Americans in the hospital with COVID-19 have dropped sharply in the past few weeks, but deaths are still running at close to all-time highs at an average of around 3,100 a day. Deaths often lag behind the infection curve, because it can take weeks to sicken and die from COVID-19. As the Super Bowl approaches, Fauci is warning people against inviting others over
Vaccination eligibility to be expanded starting Feb.8
Governor Kay Ivey and the Alabama Department of Public Health (ADPH) announced they will extend eligibility for COVID-19 vaccinations to include people 65 or older, and additional groups of frontline workers starting February 8. These additional groups will add over 1 million people who are now eligible to receive the Covid-19 vaccine in Alabama. Two million people will be eligible to get the vaccine, while only around 100,000 doses are being distributed weekly. The list of frontline critical workers includes first responders, corrections officers, food and agriculture workers, postal workers, grocery workers, public transit workers, judiciary, and educational personnel (teachers, support staff, community college, and higher education). Recently the Alabama Education Association sent a letter to State Health Officer Dr. Scott Harris, asking the state to begin vaccinating education employees “as soon as possible.” 39 school workers in Alabama have died from COVID-19. 772,275 vaccines have been delivered to Alabama, and 42% of those have already been administered. 148,549 doses of the Pfizer vaccine and 175,326 doses of the Moderna vaccine have been used. Gov. Ivey stated, “We have all been frustrated that the supply of vaccine coming from the federal government hasn’t kept up with the demand. To be blunt, we simply haven’t gotten the vaccine that we’ve been promised, and this has created a major backlog of aggravation. Today’s announcement will ensure that as more vaccine is released, we will have a plan in place to get the vaccine in people’s arms more quickly.” “Alabama is expanding its guidance despite the limited vaccine in order to accelerate the vaccine uptake in our state,” Dr. Harris commented. “I want to reiterate that any remaining vaccines that have not been administered are either someone’s first dose and they are waiting on their appointment or they are waiting on their second dose. Any vaccine currently in the state has someone’s name on it.” To schedule an appointment for the free COVID-19 vaccination at a county health department, individuals may call the ADPH COVID-19 Vaccine Scheduling Hotline at 1-855-566-5333. The vaccine providers can be found within the Alabama COVID-19 Vaccine Distribution Dashboard at arcg.is/OrCey. The Vaccine Allocation Plan is available at www.alabamapublichealth.gov/covid19vaccine/assets/adph-covid19-vaccination-allocation-plan.pdf. The ADPH dashboard can be viewed here.
Dan Sutter: Are we paying twice for COVID medicines?

Two vaccines appear highly effective against SARS-CoV-2, and remdesivir is helping doctors treat severe COVID cases. These products raise challenging questions regarding patents and government funding of research. If taxpayers fund a medical breakthrough, should we then have to pay for the medicine? Consider Gilead Science’s remdesivir, which effectively treated COVID in a clinical trial. The National Institutes of Health (NIH) and the Defense Department funded the drug’s development. Public Citizen estimates that public funding totals at least $70 million. They argue that remdesivir should be priced at cost because taxpayers “should not have to pay twice” for it. Before addressing this question, let’s consider the rationale for patents. Patents help ensure the funding of research producing knowledge. Medicines and vaccines are ultimately knowledge that a given combination of chemicals keeps us from getting sick or restores our health. Research must be performed to generate new knowledge and is highly uncertain; experiments do not always yield breakthroughs. The prices of successful medicines and vaccines must cover the cost of all this research. Knowledge is challenging to market. Imagine trying to sell some new fact which nobody else knows. The first person buying this new fact can then sell it to others, undercutting the price you need to charge to recover your research costs. For a medicine, a chemist can analyze a sample to determine the formula, while scientists can reverse-engineer new products. Patents provide inventors exclusive rights to their inventions for a limited number of years. The patent prohibits copying of the invention. Ideally, the patent should be only long enough to encourage research, but calculations cannot be made with precision. Both businesses and the Federal government fund research. The NIH spends $40 billion a year on medical research. Successful medical researchers typically must win NIH grants. The public-private division of research is in principle along the line between basic and applied research. Business funding typically becomes available when knowledge is close to yielding a marketable product; this is applied research, or the development part of R&D. Basic research advances knowledge for knowledge’s sake. Although as fundamental breakthroughs percolate through society, people begin to recognize the practical applications, the commercial value of basic research is frequently too remote to attract investors. We can see how taxpayers could “pay twice” for new medicines. If a new medicine were developed almost entirely through government funding, the company marketing the drug needs profits to recover its negligible research expenses. Most medicines though – and new products generally – require a mix of research and development. Even if the Federal government funds the basic research, a lot of work remains to yield a commercially valuable product. The Moderna and BioNTech SARS-CoV-2 vaccines illustrate this. Both use messenger RNA vaccines, and the NIH funded the basic research on m-RNA. The breakthrough has offered enormous promise for 25 years but prior to 2020 no medicines on the market. Turning m-RNA into medicine involved further breakthroughs to encase bioengineered proteins in lipid nanoparticles. Moderna and BioNTech have done much of this last portion of the research. What about the estimated $10 billion in Federal spending on COVID vaccines through Operation Warp Speed? This has covered the testing and production of vaccines. Manufacturing is distinct from the knowledge contained in a candidate vaccine, and patents reward knowledge creation. Knowledge drives our prosperity. More important than the mix of public versus private sector research is the discovery of new knowledge. COVID-19 has revealed a very healthy global biomedical research industry. Remdesivir and other treatments have reduced the fatality rate among hospitalized patients by an estimated 70 percent since March. And researchers formulated two seemingly highly effective vaccines within weeks of the identification of the novel virus’ DNA. Taxpayers should not have to pay twice for the same medicine. Yet politicians have protected health at enormous cost during the pandemic. Overpaying for effective vaccines or medicines is frustrating but preferable to going without. Daniel Sutter is the Charles G. Koch Professor of Economics with the Manuel H. Johnson Center for Political Economy at Troy University and host of Econversations on TrojanVision. The opinions expressed in this column are the author’s and do not necessarily reflect the views of Troy University.
Coronavirus vaccine will be free for all Alabamians, available as soon as December
With two possible vaccines being released as soon as December, the Alabama Department of Health announced that the COVID-19 vaccine will be free for all Alabamians. According to the press release, healthcare providers and the chronically ill would be the first to receive the vaccine. Residents and workers in long-term care facilities will also be prioritized. Drug company Moderna announced their vaccine has a 94% effectiveness rate, AL.com reported. This is slightly higher effectiveness than Pfizer’s vaccine, which has a 90% effectiveness. Most vaccines will require two doses taken three to four weeks apart. Dozens of companies have vaccines in the works. There are already large doses of the Pfizer and Moderna vaccines being produced and stored. U.S. Army Gen. Gustave Perna, Chief Operating Officer of Operation Warp Speed, recently spoke with State Health Officer Dr. Scott Harris about how quickly Alabama will receive the vaccinations. The FDA is expecting to spend around two weeks reviewing the vaccine trials before beginning distribution. Once the review is complete, the Advisory Committee on Immunization Practices will study the data and make recommendations. Once it is confirmed that the vaccine is safe and effective, and when an Emergency Use Authorization is received, vaccine kits will be shipped to several locations statewide. “All Americans can receive their initial vaccine treatment without any charge, including people with no health insurance. Gen. Perna and State Health Officer Dr. Scott Harris emphasized that the goal is for all people to have access to the free vaccine regardless of their financial status or location. Distribution of the vaccine will be made equitably to those most at risk, the chronically ill and seniors in all 67 Alabama counties.”


