First over-the-counter birth control pill gets FDA approval
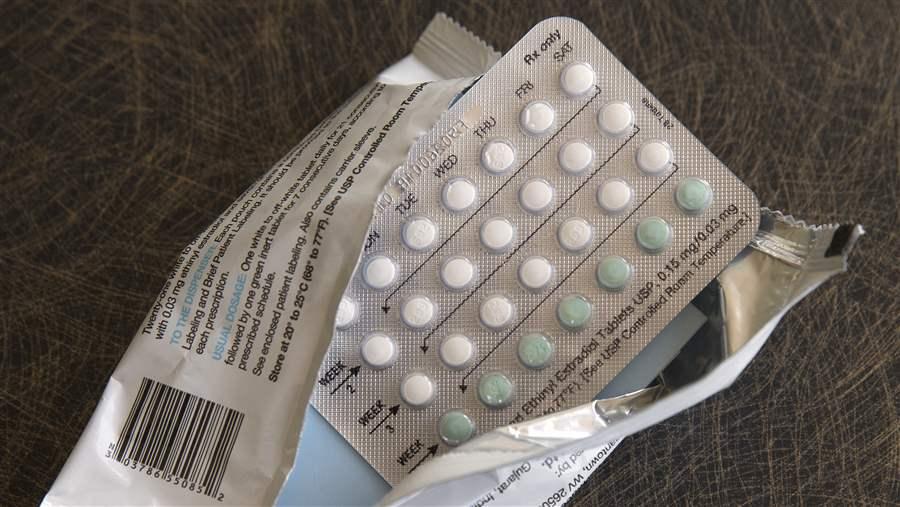
Federal regulators on Thursday approved the nation’s first over-the-counter birth control pill in a landmark decision that will soon allow American women and girls to obtain contraceptive medication as easily as they buy aspirin and eyedrops. The Food and Drug Administration cleared once-a-day Opill to be sold without a prescription, making it the first such medication to be moved out from behind the pharmacy counter. The manufacturer, Ireland-based Perrigo, won’t start shipping the pill until early next year, and there will be no age restrictions on sales. Hormone-based pills have long been the most common form of birth control in the U.S., used by tens of millions of women since the 1960s. Until now, all of them required a prescription. Medical societies and women’s health groups have pushed for wider access for decades, noting that an estimated 45% of the 6 million annual pregnancies in the U.S. are unintended. Teens and girls, women of color, and those with low incomes report greater hurdles in getting prescriptions and picking them up. The challenges can include paying for a doctor’s visit, getting time off from work, and finding child care. “This is really a transformation in access to contraceptive care,” said Kelly Blanchard, president of Ibis Reproductive Health, a nonprofit group that supported the approval. “Hopefully, this will help people overcome those barriers that exist now.” Perrigo says Opill could be an important new option for the estimated 15 million U.S. women who currently use no birth control or less effective methods, such as condoms. They are a fifth of women who are child-bearing age. But how many women will actually gain access depends on the medication’s price, which Perrigo plans to announce later this year. “The reason why so many of us worked tirelessly for years to get over-the-counter birth control pills is to improve access … cost shouldn’t be one of those barriers,” said Dr. Pratima Gupta of the American College of Obstetricians and Gynecologists. Most older birth control pills cost $15 to $30 for a month’s supply without insurance coverage. Over-the-counter medicines are generally much cheaper than prescriptions, but they typically aren’t covered by insurance. Forcing insurers to cover over-the-counter birth control would require a regulatory change by the federal government, which women’s advocates are urging the Biden administration to implement. The FDA approval gives U.S. women another birth control option amid the legal and political battles over reproductive health, including last year’s reversal of Roe v. Wade, which has upended abortion access across the U.S. That said, Opill’s approval is unrelated to the ongoing court battles over the abortion pill mifepristone. And anti-abortion groups have generally emphasized that they do not oppose contraceptives, which are used to prevent pregnancies, not end them. However, that has done little to ease fears that contraception could someday become a target. When the Supreme Court overturned Roe, Justice Clarence Thomas wrote a separate opinion in which he explicitly called on his colleagues to put the high court’s same-sex marriage, gay sex, and contraception cases on the table. In the last year, the FDA has faced pressure from Democratic politicians, health advocates, and medical professionals to improve access to birth control. The American Medical Association and other leading medical groups backed Opill’s application for over-the-counter status. Birth control pills are available without a prescription across much of South America, Asia, and Africa. Perrigo submitted years of research to the FDA to show that women could understand and follow instructions for using the pill. Thursday’s approval came despite some concerns by FDA scientists about the company’s results, including whether women with certain underlying medical conditions would understand that they shouldn’t take the drug. The FDA’s action only applies to Opill. It’s in an older class of contraceptives, sometimes called minipills, that contain a single synthetic hormone and generally carry fewer side effects than more popular combination hormone pills. Women’s health advocates hope the decision paves the way for more over-the-counter birth control options and, eventually, for abortion pills to do the same. An outside panel of FDA advisers unanimously voted in favor of the switch at a hearing in May where dozens of public speakers called for Opill’s approval. Dyvia Huitron was among those who presented, explaining how she has been unable to get prescription birth control more than three years after becoming sexually active. The 19-year-old University of Alabama student said she still isn’t comfortable getting a prescription because the school’s health system reports medical exams and medications to parents. “My parents did not let me go on the pill,” Huitron said in a recent interview. “There was just a lot of cultural stigma around being sexually active before you’re married.” While she uses other forms of contraception, “I would have much preferred to have birth control and use these additional methods to ensure that I was being as safe as possible.” Advocates were particularly interested in Opill because it raised fewer safety concerns. The pill was first approved in the U.S. five decades ago. “It’s been around a long time, and we have a large amount of data supporting that this pill is safe and effective for over-the-counter use,” Blanchard said. Newer birth control pills typically combine two hormones, estrogen, and progestin, which can help make periods lighter and more regular. But their use carries a heightened risk of blood clots, and they shouldn’t be used by women at risk for heart problems, such as those who smoke and are over 35. Opill has only progestin, which prevents pregnancy by blocking sperm from reaching the cervix. It must be taken around the same time daily to be most effective. In its internal review published in May, the FDA noted that some women in Perrigo’s study had trouble understanding the drug’s labeling information. In particular, the instructions warn that women with a history of breast cancer should not take the pill because it could spur tumor growth. Common side effects include irregular vaginal bleeding, headaches, dizziness, and cramps, according to the FDA. The label also cautions that certain drugs can interfere with
Cost of over-the-counter Narcan could be barrier to use

The U.S. Food and Drug Administration’s decision to give an opioid overdose antidote over-the-counter status could help as illicit fentanyl floods of the nation’s illegal drug market, but how helpful it will be could depend on how much it will cost. The U.S. Food and Drug Administration’s decision means sales of Narcan nasal spray would soon be allowed at pharmacies, grocery stores, convenience stores, gas stations, and online. Narcan, a naloxone product, is a medication that rapidly reverses the effects of opioid overdose. Naloxone is the standard treatment for an opioid overdose. Emergent BioSolutions Inc., which makes Narcan, said that the nasal spray will be available on U.S. shelves and at online retailers by the late summer. The company said it will have to account for “manufacturing changes that will be implemented to support nonprescription packaging” and “supply chain modifications.” It has not yet said how much the drug will cost. FDA Commissioner Dr. Robert Califf said in a statement that the agency was encouraging Emergent BioSolutions to make access to the product at an affordable price a priority. How much it will cost could determine how effective it is at reducing overdose deaths, said Evan Peet, an economist at the RAND Corporation. While making the drug available without a prescription could help reduce one barrier to access, price could be another barrier, especially for addicts. The FDA decision is “likely good for some groups, bad for others, and still uncertain for some,” Peet said. Making Narcan available without a prescription could increase costs for people with insurance, particularly those with Medicaid, the government program that provides health coverage to low-income people. “Typically, if something is available over-the-counter, then it is not covered by insurance,” Peet said. People with private insurance typically pay $10 to $30 for naloxone, while those with Medicaid or Medicare pay even less, about $2 to $3 for naloxone. For those without insurance, the current price is much higher, about $250 on average, according to the most recent estimates available from 2018. Groups have forecasted the estimated over-the-counter price for the drug will range from $35 to $65. Emergent BioSolutions did not respond to requests about pricing information or when such information would be available. “Disproportionately, people with opioid use disorder are uninsured,” Peet said. “And so maybe [over-the-counter] is going to be a net benefit because those people are potentially the most vulnerable. But also, people with Medicaid have lower income and are more price sensitive, so it may be particularly negative for that group.” Illicit fentanyl, which is more potent than heroin and some other opioids, poses additional challenges. “Fentanyl is really driving the bus now,” Peet said. Because of its potency, fentanyl overdoses may require more than one dose of Narcan. Adulterants, such as the veterinary tranquilizer xylazine, pose additional challenges. Earlier this month, the U.S. Drug Enforcement Administration issued a warning about a sharp increase in trafficking of fentanyl mixed with xylazine. The federal agency said xylazine and fentanyl mixtures had been seized in 48 of 50 states. It also noted that the DEA Laboratory System reported that in 2022 about 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine. “Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” DEA Administrator Anne Milgram said in a statement. U.S. officials reported 107,735 overdose deaths between August 2021 and August 2022 from drug poisonings, according to the U.S. Centers for Disease Control and Prevention. About 66% of those deaths involved synthetic opioids such as fentanyl. Republished with the permission of The Center Square.
Missouri leads 20 AGs warning Walgreens, CVS about mailing abortion pills
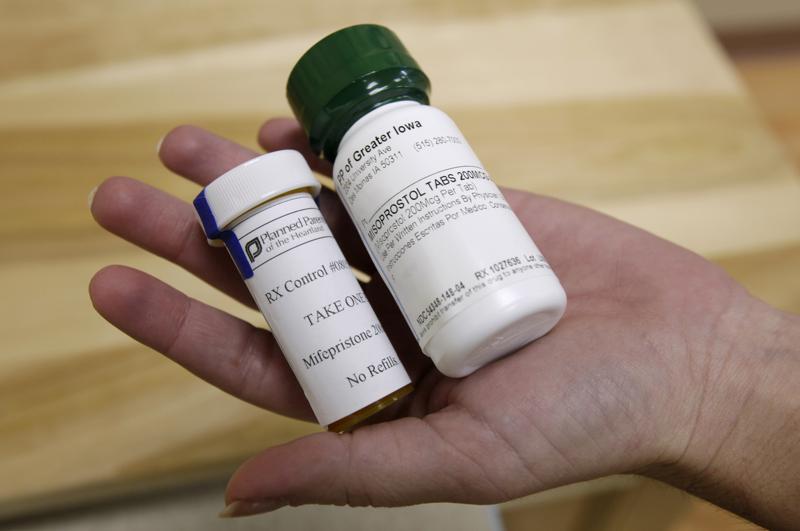
Republican Missouri Attorney General Andrew Bailey and 19 other attorneys general are warning Walgreens and CVS pharmacies that any plans to mail abortion-inducing pills is illegal and unsafe. “As the principal legal and law enforcement officers of our 20 states, we offer you these thoughts on the current legal landscape,” Bailey wrote. In late January, the Food and Drug Administration approved mifepristone to be used in a regimen with misoprostol “to end an intrauterine pregnancy through 10 weeks gestation.” The drugs must be dispensed or under the supervision of a certified prescriber or pharmacy and “may be dispensed in-person or by mail,” according to the FDA website. In late December, the Department of Justice published a 21-page memorandum for the U.S. Postal Service stating “no matter where the drugs are delivered, a variety of uses of mifepristone and misoprostol serve important medical purposes and are lawful under federal and state law.” The document said the USPS couldn’t assume the drugs can’t be mailed because “they are being sent into a jurisdiction that significantly restricts abortion.” CVS and Walgreens recently announced they are seeking certification from the FDA to sell and mail the pills, according to multiple media reports. “As Attorney General, it is my responsibility to enforce the laws as written, and that includes enforcing the very laws that protect Missouri’s women and unborn children,” Bailey said in a statement. “My office is doing everything in its power to inform these companies of the law, with the promise that we will use every tool at our disposal to uphold the law if broken.” Missouri became the first state to end elective abortions when a “trigger law” was executed hours after the U.S. Supreme Court overturned Roe v. Wade last June. In addition to prohibiting doctors from performing abortions unless there is a medical emergency, Missouri law states, “any person who knowingly performs or induces an abortion of an unborn child in violation of this subsection shall be guilty of a class B felony, as well as subject to suspension or revocation of his or her professional license by his or her professional licensing board.” Bailey’s letter to the companies stated Missouri law prohibits “using the mail to send or receive abortion drugs.” Bailey referred to research published by Advancing New Standards in Reproductive Health (ANSRH) stating medication abortions were 5.96 times as likely to result in a complication as first-trimester aspiration abortions. (When the Supreme Court ruled last June, ANSRH stated the decision contradicted scientific evidence and said it “stands against this decision as one that will have devastating consequences to people’s lives and their families.”) “And finally,” Bailey wrote, “mail-order abortion pills also invite the horror of an increase in coerced abortions. When abortion drugs are mailed or consumed outside a regulated medical facility, the risk of coercion is much higher – indeed, guaranteed – because there is no oversight. Outside the regulated medical context, a person can obtain an abortion pill quite easily and then coerce a woman into taking it.” The attorneys general from Alabama, Alaska, Arkansas, Florida, Georgia, Indiana, Iowa, Kentucky, Louisiana, Mississippi, Montana, North Dakota, Ohio, Oklahoma, South Carolina, South Dakota, Texas, Utah, and West Virginia signed Bailey’s letter. Republished with the permission of The Center Square.
Joe Biden invokes Defense Production Act for formula shortage

President Joe Biden on Wednesday invoked the Defense Production Act to speed production of infant formula and authorized flights to import supply from overseas as he faces mounting political pressure over a domestic shortage caused by the safety-related closure of the country’s largest formula manufacturing plant. The Defense Production Act order requires suppliers of formula manufacturers to fulfill orders from those companies before other customers, in an effort to eliminate production bottlenecks. Biden is also authorizing the Defense Department to use commercial aircraft to fly formula supplies that meet federal standards from overseas to the U.S., in what the White House is calling “Operation Fly Formula.” Supplies of baby formula across the country have been severely curtailed in recent weeks after a February recall by Abbott Nutrition exacerbated ongoing supply chain disruptions among formula makers, leaving fewer options on store shelves and increasingly anxious parents struggling to find nutrition for their children. “I know parents across the country are worried about finding enough formula to feed their babies,” Biden said in a video statement released by the White House. ”As a parent and as a grandparent, I know just how stressful that is.” The announcement comes two days after the Food and Drug Administration said it was streamlining its review process to make it easier for foreign manufacturers to begin shipping more formula into the U.S. In a letter Wednesday to the Department of Health and Human Services and the Department of Agriculture, Biden directed the agencies to work with the Pentagon to identify overseas supply of formula that meets U.S. standards over the next week so that chartered Defense Department flights can swiftly fly it to the U.S. “Imports of baby formula will serve as a bridge to this ramped-up production,” Biden wrote. Regulators said Monday that they’d reached a deal to allow Abbott Nutrition to restart its Sturgis, Michigan, plant, the nation’s largest formula plant, which has been closed since February due to contamination issues. The company must overhaul its safety protocols and procedures before resuming production. After getting the FDA’s OK, Abbott said it will take eight to ten weeks before new products begin arriving in stores. The company didn’t set a timeline to restart manufacturing. “I’ve directed my team to do everything possible to ensure there’s enough safe baby formula and that it is quickly reaching families that need it the most,” Biden said in the statement, calling it “one of my top priorities.” The White House actions come as the Democratic-led House approved two bills Wednesday addressing the baby formula shortage as lawmakers look to show progress on what has become a frightening development for many families. One bill with wide bipartisan support passed by a vote of 414-9. It would give the secretary of the Agriculture Department the ability to issue a narrow set of waivers in the event of a supply disruption. The goal is to give participants in an assistance program commonly known as WIC the ability to use vouchers to purchase formula from any producer rather than be limited to one brand that may be unavailable. The WIC program accounts for about half of infant formula sales in the U.S. “I want to say to the mom struggling that we hear you in Congress, and you do not need to handle this on your own. We are working to find you a solution,” said the bill’s sponsor, Rep. Jahana Hayes, D-Conn. The other measure, a $28 million emergency spending bill to boost resources at the Food and Drug Administration, passed by a mostly party-line vote of 231-192, and it’s unclear whether the Senate will go along. “This bill just continues the Democrats’ strategy of throwing money at the same bureaucrats who caused the crisis and who have not made its solution a priority,” said Rep. Andy Harris, R-Md. Rep. Rosa DeLauro, the Democratic chair of the House Appropriations Committee, said the money would increase FDA staffing to boost inspections of domestic and international suppliers, prevent fraudulent products from getting onto store shelves and acquire better data on the marketplace. “It is essential that we ensure the federal government has the resources it needs to get baby formula back on the shelves,” said House Speaker Nancy Pelosi, D-Calif. Abbott’s voluntary recall was triggered by four illnesses reported in babies who had consumed powdered formula from its plant. All four infants were hospitalized with a rare type of bacterial infection, and two died. After a six-week inspection, FDA investigators published a list of problems in March, including lax safety and sanitary standards and a history of bacterial contamination in several parts of the plant. Under Monday’s agreement, Abbott must regularly consult with an outside safety expert to restart and maintain production. Chicago-based Abbott has emphasized that its products have not been directly linked to bacterial infections in children. Samples of the bacteria found at its plant did not match the strains collected from two babies by federal investigators. But FDA officials pushed back on that reasoning Monday on a call with reporters — their first time publicly addressing the company’s argument. FDA staffers noted they were unable to collect bacterial strains from two of the four patients, limiting their chances of finding a match. “Right from the get-go, we were limited in our ability to determine with a causal link whether the product was linked to these four cases because we only had sequences on two,” FDA’s food director Susan Mayne said. Fixing the violations uncovered at Abbott’s plant will take time, according to former FDA officials. Companies need to exhaustively clean the facility and equipment, retrain staff, repeatedly test and document that there is no contamination. As part of the FDA’s new import policy, regulators said companies would need to provide documentation of their factory’s inspections. Republished with the permission of the Associated Press.
Abbott says agreement reached to reopen baby formula plant

Baby formula maker Abbott said Monday it has reached an agreement with U.S. health regulators to restart production at its largest domestic factory, though it will be well over a month before any new products ship from the site to help alleviate the national shortage facing parents. Abbott did not immediately detail the terms of the agreement with the Food and Drug Administration, which has been investigating safety concerns at the Sturgis, Michigan, plant since early this year. The consent decree amounts to a legally binding agreement between the FDA and the company on the steps needed to reopen the factory. An FDA spokeswoman did not immediately respond to a request for comment on the announcement Monday evening. After production resumes, Abbott said it will take between six-to-eight weeks before new products begin arriving in stores. The company didn’t set a timeline to restart production. The FDA is expected to announce additional steps Monday to allow more foreign imports into the U.S. to address the supply problems. It comes as the administration of President Joe Biden faces intense pressure to do more to ease the shortage that has left many parents hunting for formula online or at food banks. Abbott’s plant came under scrutiny early this year after the FDA began investigating four bacterial infections among infants who consumed powdered formula from the plant. Two of the babies died. In February, the company halted production and recalled several brands of powdered formula, squeezing supplies that had already been tightened by supply chain disruptions and stockpiling during COVID-19. The shortage has led retailers like CVS and Walgreen’s to limit how many containers customers can purchase per visit. Outrage over the issue has quickly snowballed and handed Republicans a fresh talking point to use against Biden ahead of November’s midterm elections. Abbott is one of just four companies that produce roughly 90% of U.S. formula, and its brands account for nearly half that market. After a six-week inspection, FDA investigators published a list of problems in March, including lax safety and sanitary standards and a history of bacterial contamination in several parts of the plant. Chicago-based Abbott has emphasized that its products have not been directly linked to the bacterial infections in children. Samples of the bacteria found at its plant did not match the strains collected from the babies by federal investigators. The company has repeatedly stated it is ready to resume manufacturing, pending an FDA decision. Former FDA officials say fixing the type of problems uncovered at Abbott’s plant takes time, and infant formula facilities receive more scrutiny than other food facilities. Companies need to exhaustively clean the facility and equipment, retrain staff, and repeatedly test and document that there is no contamination. On Monday, FDA Commissioner Robert Califf told ABC News that an announcement was forthcoming about importing baby formula from abroad. The key issue is making sure the instructions for the formula are in languages that mothers and caregivers can understand, he noted. Pediatricians say baby formulas produced in Canada and Europe are roughly equivalent to those in the U.S. But traditionally, 98% of the infant formula supply in the U.S. is made domestically. Companies seeking to enter the U.S. face several major hurdles, including rigorous research and manufacturing standards imposed by the FDA. San Diego father Steven Davis has faced heart-wrenching challenges finding formula for his medical fragile daughter, who was on an Abbott formula but has had to switch with the recall and subsequent shortages in other brands. Zoie Davis was born 19 months ago with no kidneys, a rare, life-threatening condition that requires dialysis and a feeding tube until she weighs enough for a kidney transplant. She’s four pounds shy of that milestone, said Davis, a mortgage lender and his daughter’s caretaker. “Her life is dependent on her weight gain,” he said. Davis said he used an organic brand from overseas until costs and customs hurdles made that too difficult. Friends and strangers from out of state have sent him other brands, but each time she switches requires more blood tests and monitoring, Davis said. Despite her challenges, Zoie is walking, talking, and “doing pretty good” on other developmental milestones, Davis said. “She’s a shining light in my life,” he said. Republished with the permission of the Associated Press.
FDA authorizes 1st breath test for COVID-19 infection

The Food and Drug Administration on Thursday issued an emergency use authorization for what it said is the first device that can detect COVID-19 in breath samples. The InspectIR COVID-19 Breathalyzer is about the size of a piece of carry-on luggage, the FDA said, and can be used in doctor’s offices, hospitals, and mobile testing sites. The test, which can provide results in less than three minutes, must be carried out under the supervision of a licensed health care provider. Dr. Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, called the device “yet another example of the rapid innovation occurring with diagnostic tests for COVID-19.” The FDA said the device was 91.2% accurate at identifying positive test samples and 99.3% accurate at identifying negative test samples. “InspectIR expects to be able to produce approximately 100 instruments per week, which can each be used to evaluate approximately 160 samples per day,” the agency said. “At this level of production, testing capacity using the InspectIR COVID-19 Breathalyzer is expected to increase by approximately 64,000 samples per month.” Republished with the permission of the Associated Press.
Lindsey Stroud: No, the FDA does not need to regulate cannabis

The U.S. House of Representatives passed the Marijuana Opportunity Reinvestment and Expungement (MORE) Act on April Fool’s Day. This non-foolish comprehensive cannabis-reform bill would remove cannabis from scheduled drug classification under the Controlled Substance Act, expunge previous cannabis-related convictions, and establish a federal tax rate on all cannabis products in the states. Federal decriminalization and even legalization of cannabis is no joke, but there are some in Congress who think that the federal government needs more regulatory authority. The bill, as currently written, charges that “the Secretary of Health and Human Services, acting through the Commissioner of Food and Drugs, shall hold not less than one public meeting to address the regulation, safety, manufacturing, product quality, marketing, labeling, and sale of product containing cannabis or cannabis-derived compounds.” Essentially, under the MORE Act, all cannabis products could (if not, would) be regulated by the U.S. Food and Drug Administration (FDA). And unfortunately, as proven time and time again, consumer products and innovation languishes once under FDA regulations. The history of the FDA dates to the 1906 Food and Drug Act and essentially morphed into the 1938 Food, Drug and Cosmetic (FD&C) Act, which the agency still operates under today. As of March 15, 2022, the FD&C Act is 871 pages of bloated bureaucratic privileges afforded to an agency that has long suppressed innovation across many sectors of consumer products. Currently, there is no mention of cannabis at all in FDA regulations. One doesn’t need to look too far in FDA regulations to understand that the agency is more of a barrier to rather than a supporter of innovation and has applied disastrous rules that harm small businesses. For example, under the FDA’s Center for Tobacco Products, all new tobacco products that enter the market after 2007 must undergo an extensive approval process to even market their products. This has significantly lessened adult access to tobacco harm reduction products, despite these products being promoted by public health agencies in other countries. Moreover, in September 2021, the agency actually issued denial orders to nearly 1 million vapor products that had been manufactured by small e-cigarette firms since 2016. The FDA’s Center for Drug Evaluation and Research (CDER) oversees drug approval. Bringing a new drug to market is expensive and takes time. In fact, some studies have shown that bringing a new drug to market can cost more than $1 billion and take more than a decade. The Center for Food Safety and Applied Nutrition is the FDA branch that oversees about 78 percent of the food consumed by Americans. With the 2011 Food Safety and Modernization Act of 2011, Congress gave the FDA “expanded authority to regulate fresh-produce production at the farm level.” Unfortunately, as noted in a 2018 study by the U.S. Department of Agriculture, the fixed costs in the certain requirements of the 2011 Act meant that “compliance costs to be higher as a share of revenue for smaller firms.” These are just a few snippets of the massive authority FDA already has over consumer products and the costs associated with FDA-created regulations. Should the FDA oversee the regulation of cannabis products, many small firms could be shut out of what is already a legal market in their respective state. While the language of the MORE Act does not have specific regulations in place, small businesses in America have already faced regulations imposed by the FDA on existing and legal consumer products. In 2009, the FDA tried to block shipments of e-cigarettes to the U.S using their CDER regulatory authority and was promptly sued by e-cigarette manufacturers. Also, in 2019, Congress gave the FDA authority to regulate tobacco products, effectively creating the CTP. In 2012, the original 2009 litigation would be finalized with the U.S. Court of Appeals for the D.C. Circuit determining that the FDA could regulate e-cigarettes as a tobacco product. In 2016, the FDA established the long-awaited deeming regulations, codifying federal regulations for novel tobacco products, including any that had entered the market after February 15, 2007 – or two years prior to FDA regulatory authority over tobacco products. As of March 2022, only two companies had received marketing orders from the agency for their e-cigarette products, while many more have been issued denials. The absence of FDA regulation has permitted other adult consumer goods to flourish. For example, the craft beer market has blossomed across America. In 2012, two companies controlled 90 percent of the American beer market, but between 2008 and 2016, breweries “expanded by a factor of six, and the number of brewery workers grew by 120 percent.” Further, between 2007 and 2016, “shipments from five major brewers … fell by 14 percent.” In 2021, even amid the COVID-19 pandemic, craft beer sales grew by eight percent, “raising small and independent brewers’ share of the U.S. beer market by volume to 13.1%.” As noted, the FDA has not been the best federal agency in empowering small businesses. In fact, its regulations tend to favor large corporations at the expense of the small businesses who created the market. Some in the cannabis space are paying attention to the actions by the FDA. Due to a lack of federal regulations, “only bold, small players have entered the hemp and hemp CBD space,” and as of October 2021, no “Fortune-500 type corporations have entered [the market] because a fear of regulatory uncertainty.” In fact, the MORE Act could “herald a major shakeup as well-funded, and well-positioned corporations with comprehensive distribution networks would replace smaller hemp companies.” Thankfully, the MORE Act isn’t the only comprehensive cannabis reform bill before Congress. Representative Nancy Mace (R-S.C.) has introduced the States Reform Act, which decriminalizes marijuana, sets a lower tax rate than the MORE Act, and, more importantly, establishes a federal regulatory scheme that is similar to alcohol. The FDA does a questionable job at best regulating adult consumer products. There is also overwhelming evidence that FDA regulations snuff out small businesses, even established businesses. Congress should avoid giving this agency
FDA halts use of antibody drugs that don’t work vs. omicron
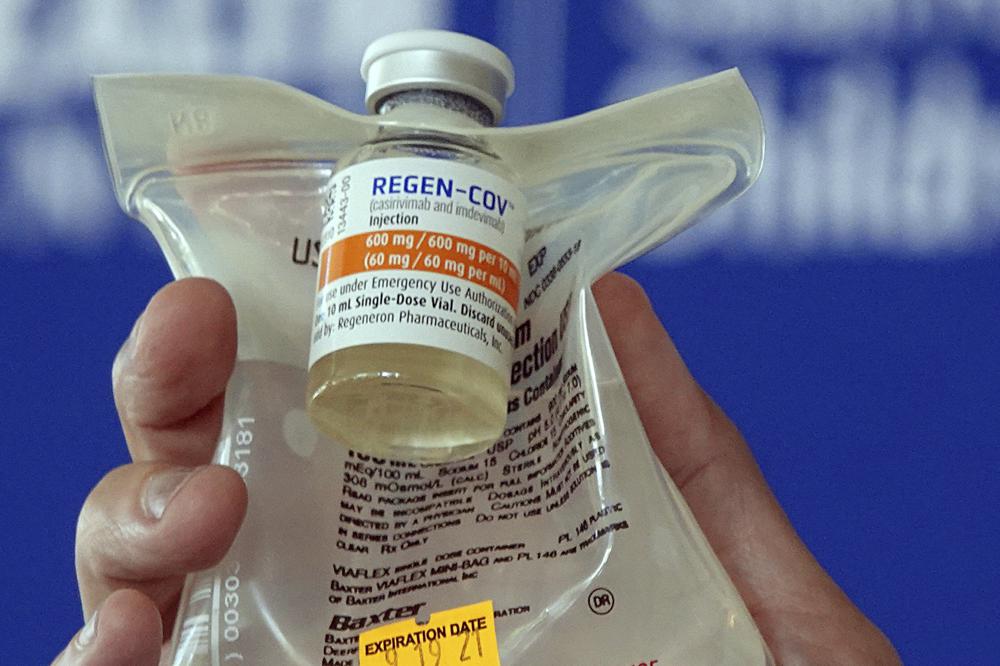
COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that now accounts for nearly all U.S. infections, U.S. health regulators said Monday. The Food and Drug Administration said it was revoking emergency authorization for both drugs, which were purchased by the federal government and have been administered to millions of Americans with COVID-19. If the drugs prove effective against future variants, the FDA said it could reauthorize their use. The regulatory move was expected because both drugmakers had said the infusion drugs are less able to target omicron due to its mutations. Still, the federal action could trigger pushback from some Republican governors who have continued promoting the drugs against the advice of health experts. Omicron’s resistance to the two leading monoclonal antibody medicines has upended the treatment playbook for COVID-19 in recent weeks. Doctors have alternate therapies to battle early COVID-19 cases, including two new antiviral pills from Pfizer and Merck, but both are in short supply. An antibody-drug from GlaxoSmithKline that remains effective also is in short supply. The drugs are laboratory-made versions of virus-blocking antibodies. They are intended to head off severe disease and death by supplying concentrated doses of one or two antibodies early in an infection. Then-President Donald Trump received Regeneron’s antibody combination after he tested positive for the coronavirus in 2020. The FDA noted in its decision that omicron accounts for more than 99% of U.S. infections, making it “highly unlikely” the antibodies would help people now seeking treatment. The agency said restricting their use would also eliminate unnecessary drug side effects, including allergic reactions. The U.S. government temporarily stopped distributing the two drugs in late December, as omicron was racing across the country to become the dominant variant. But officials resumed distribution after complaints from Republican governors, including Florida’s Ron DeSantis, who claimed that the drugs continued to help some omicron patients. DeSantis has heavily promoted antibody drugs as a signature part of his administration’s COVID-19 response, setting up infusion sites and lauding them at news conferences while opposing vaccine mandates and other public health measures. Texas Gov. Greg Abbott has also launched state-sponsored infusion sites. The drugs are not a substitute for vaccination and are generally reserved for people who are the most vulnerable, including seniors, transplant recipients, and those with conditions like heart disease and diabetes. Since early January, the U.S. government has shipped enough doses of the two antibodies to treat more than 300,000 patients. Both Regeneron and Lilly previously announced they were developing new antibodies that target omicron. The move comes days after regulators broadened the use of remdesivir — the first drug approved for COVID-19 — to treat more patients. On Friday, the FDA expanded the antiviral’s approval to include adults and children with early COVID-19 who face a high risk of ending up in the hospital. Remdesivir previously had been limited to hospitalized patients. An influential panel of federal experts had already recommended using the infused drug to try to head off hospitalization. The same guidelines from the National Institutes of Health panel recommend against the continued use of Lilly and Regeneron’s antibody drugs due to their reduced effectiveness against omicron. Still, many hospitals will face challenges in ramping up remdesivir treatments. The drug requires three consecutive IV infusions over three days when used for nonhospitalized patients. That time-consuming process won’t be an option for many over-capacity hospitals facing staff shortages. The FDA made its decision based on a 560-patient study that showed a nearly 90% reduction in hospitalizations when remdesivir is given within seven days of symptoms. The study predates the omicron variant, but, like other antivirals, remdesivir is expected to maintain its performance against the latest variant. Republished with the permission of the Associated Press.
Pfizer vaccine booster dose authorized for children 12-15 years old
The Alabama Department of Public Health (ADPH) announced new Covid-19 vaccine recommendations from the Centers for Disease Control and Prevention (CDC). The CDC has authorized the use of the Pfizer-BioNTech vaccine as a single booster dose in individuals 12 through 15 years of age. Previously, the booster dose of the vaccine was authorized for adolescents 16 years of age and older. The CDC also approved a third primary series dose of the Pfizer-BioNTech vaccine. The third dose can be administered at least 28 days following the two-dose regimen in individuals 5 through 11 years of age who are determined to be moderately to severely immunocompromised. The updates came after an ongoing review of the available safety and efficacy data by the Food and Drug Administration (FDA). Any known or potential benefits of additional doses were determined to outweigh any potential vaccine risks. The CDC also lowered the authorized dosing interval of a booster dose to at least five months after completing a Pfizer-BioNTech or Moderna primary series. The booster dose after the Johnson and Johnson vaccine remains at two months. The Moderna and Johnson & Johnson/Janssen vaccines are only authorized for adults 18 years of age and older. During a press conference last week, ADHP health officer Dr. Scott Harris expressed concern for Alabamans and the sudden rise in omicron variant cases. The Covid-19 positivity rate in Alabama is almost 39%. That means nearly four in 10 tests came back positive. “It will infect everyone in the state at some point, or most of them,” Harris stated. “So, we really need people to do the single most important thing they can do to protect themselves, which is to be fully vaccinated and boosted when it’s appropriate to do that.” Alabama currently has one of the nation’s lowest vaccination rates. According to the CDC, less than 48% of the state’s population is fully vaccinated. Studies show that protection decreases over time after getting vaccinated against COVID-19 and may also be decreased due to changes in circulating variants.
Alabama to get limited supply of new COVID-19 treatment

With COVID-19 hospitalizations and cases on the rise in Alabama, the state will have a limited number of doses of a new drug that can be used to treat the illness, health officials said Thursday. The state’s initial supply of 780 courses of the Pfizer oral drug Paxlovid, which the Food and Drug Administration approved for emergency use as the omicron variant spreads rapidly, will be distributed through pharmacies, the Department of Public Health said in a statement. Dr. Scott Harris, the state health officer, said the drug will be available to people who aren’t hospitalized with the illness but isn’t a substitute for vaccinations, “which remain the best way for most people to protect themselves against severe illness and death due to COVID-19.” “I continue to urge all Alabamians to be vaccinated and receive a booster dose when eligible,” Harris said. Paxlovid, which will be available by prescription by the first week of January, was authorized for the treatment of mild-to-moderate COVID-19 symptoms in adults and children. Other states will also receive limited supplies before production and distribution increases, officials said. Less than half of the state’s population is fully vaccinated, and relatively few people are following health recommendations to wear masks in public places and maintain a safe distance from others. In Hoover earlier this week, unmasked holiday shoppers far outnumbered people with covered faces at the state’s largest shopping mall, the Riverchase Galleria. More than 16,380 people have died of COVID-19 in the state, giving Alabama the nation’s second-highest death rate from the illness caused by the coronavirus, according to researchers from Johns Hopkins University. Over the last two weeks, the rolling average number of daily new cases has increased by 348, or 64.1%, but the state ranks last in the nation for new cases per capita, according to Johns Hopkins. Hospitalizations for COVID-19 have jumped more than 70% statewide in a month to more than 430 on Wednesday, but that is still far below levels from early fall when officials said the state’s health care system was in danger of being overwhelmed by the illness. Republished with the permission of the Associated Press.
Pfizer pill becomes 1st US-authorized home COVID treatment

U.S. health regulators on Wednesday authorized the first pill against COVID-19, a Pfizer drug that Americans will be able to take at home to head off the worst effects of the virus. The long-awaited milestone comes as U.S. cases, hospitalizations and deaths are all rising, and health officials warn of a tsunami of new infections from the omicron variant that could overwhelm hospitals. The drug, Paxlovid, is a faster, cheaper way to treat early COVID-19 infections, though initial supplies will be extremely limited. All of the previously authorized drugs against the disease require an IV or an injection. An antiviral pill from Merck also is expected to soon win authorization. But Pfizer’s drug is all but certain to be the preferred option because of its mild side effects and superior effectiveness, including a nearly 90% reduction in hospitalizations and deaths among patients most likely to get severe disease. “The efficacy is high, the side effects are low, and it’s oral. It checks all the boxes,” said Dr. Gregory Poland of the Mayo Clinic. “You’re looking at a 90% decreased risk of hospitalization and death in a high-risk group — that’s stunning.” The Food and Drug Administration authorized Pfizer’s drug for adults and children ages 12 and older with a positive COVID-19 test and early symptoms who face the highest risks of hospitalization. That includes older people and those with conditions like obesity and heart disease. Children eligible for the drug must weigh at least 88 pounds (40 kilograms). The pills from both Pfizer and Merck are expected to be effective against omicron because they don’t target the spike protein where most of the variant’s worrisome mutations reside. Pfizer currently has 180,000 treatment courses available worldwide, with roughly 60,000 to 70,000 allocated to the U.S. Federal health officials are expected to ration early shipments to the hardest-hit parts of the country. Pfizer said the small supply is due to the manufacturing time — currently about nine months. The company says it can halve production time next year. The U.S. government has agreed to purchase enough Paxlovid to treat 10 million people. Pfizer says it’s on track to produce 80 million courses globally next year, under contracts with the U.K., Australia, and other nations. Health experts agree that vaccination remains the best way to protect against COVID-19. But with roughly 40 million American adults still unvaccinated, effective drugs will be critical to blunting the current and future waves of infection. The U.S. is now reporting more than 140,000 new infections daily, and federal officials warn that the omicron variant could send case counts soaring. Omicron has already whipped across the country to become the dominant strain, federal officials confirmed earlier this week. Against that backdrop, experts warn that Paxlovid’s initial impact could be limited. For more than a year, biotech-engineered antibody drugs have been the go-to treatments for COVID-19. But they are expensive, hard to produce, and require an injection or infusion, typically given at a hospital or clinic. Also, laboratory testing suggests the two leading antibody drugs used in the U.S. aren’t effective against omicron. Pfizer’s pill comes with its own challenges. Patients will need a positive COVID-19 test to get a prescription. And Paxlovid has only proven effective if given within five days of symptoms appearing. With testing supplies stretched, experts worry it may be unrealistic for patients to self-diagnose, get tested, see a physician and pick up a prescription within that narrow window. “If you go outside that window of time, I fully expect the effectiveness of this drug is going to fall,” said Andrew Pekosz, a Johns Hopkins University virologist. The FDA based its decision on company results from a 2,250-patient trial that showed the pill cut hospitalizations and deaths by 89% when given to people with mild-to-moderate COVID-19 within three days of symptoms. Less than 1% of patients taking the drug were hospitalized, and none died at the end of the 30-day study period, compared with 6.5% of patients hospitalized in the group getting a dummy pill, which included nine deaths. Pfizer’s drug is part of a decades-old family of antiviral drugs known as protease inhibitors, which revolutionized the treatment of HIV and hepatitis C. The drugs block a key enzyme which viruses need to multiply in the human body. The U.S. will pay about $500 for each course of Pfizer’s treatment, which consists of three pills taken twice a day for five days. Two of the pills are Paxlovid, and the third is a different antiviral that helps boost levels of the main drug in the body. Republished with the permission of the Associated Press.
U.S. regulators lift in-person restrictions on abortion pill

The Food and Drug Administration on Thursday permanently removed a major obstacle for women seeking abortion pills, eliminating a long-standing requirement that they pick up the medication in person. Millions of American women will now be able to get a prescription via an online consultation and receive the pills through the mail. FDA officials said a scientific review supported broadening access, including no longer limiting dispensing to a small number of specialty clinics and doctor’s offices. But prescribers will still need to undergo certification and training. Additionally, the agency said dispensing pharmacies will have to be certified. The decision is the latest shift in the polarized legal battle over medication abortion, which has only intensified amid the disruptions of the COVID-19 pandemic. It is certain to spur legal challenges and more restrictions in Republican-led states. Earlier this year, the FDA stopped enforcing the in-person requirement because of the pandemic. Under Thursday’s decision, the agency permanently dropped the 20-year-old rule, which has long been opposed by medical societies, including the American Medical Association, which say the restriction offers no clear benefit to patients. The FDA’s latest scientific review stems from a 2017 lawsuit led by the American Civil Liberties Union, which argued that the agency’s restrictions block or delay medical care, especially for people in low-income and rural communities. The ACLU hailed the elimination of the strictest requirements but said regulators should have gone further and allowed prescribing by any physician and broader pharmacy dispensing. Abortion opponents said the FDA decision would result in more drug-related side effects and complications for women. Physicians who prescribe the drug mifepristone, will have to certify that they can provide emergency care to deal with potential adverse effects, including excessive bleeding, FDA officials said Thursday. The change still means many more doctors will be able to write prescriptions, and American women will be able to fill their orders at far more pharmacies, including via online and mail-order services. The effect will vary by state. More than a dozen Republican-led states have passed measures that limit access to the pills, including outlawing delivery by mail. Increased use of mail-order abortion pills could pose a dilemma for the anti-abortion movement, given that its leaders generally say they don’t favor criminalizing the actions of women seeking abortions and because mail deliveries can be an elusive target for prosecutors. The latest policy shift comes as advocates on both sides of the abortion debate wait to see whether the conservative Supreme Court will weaken or even overturn the Roe v. Wade decision that guarantees the right to abortion nationwide. Roe’s demise would likely prompt at least 20 Republican-governed states to impose sweeping bans, while perhaps 15 states governed by Democrats would reaffirm support for abortion access. More complicated would be politically divided states, where fights over abortion laws could be ferocious. Medication abortion has been available in the United States since 2000 when the FDA first approved mifepristone to terminate pregnancies up to 10 weeks. Taken with a hormone blocker called misoprostol, it constitutes the so-called abortion pill. About 40% of all abortions in the U.S. are now done through medication — rather than surgery — and that option has become more pivotal during the COVID-19 pandemic. At the time of approval, the FDA imposed limits on how the drug could be distributed, including barring it from regular pharmacies and requiring that all doctors providing the drug undergo special certification. Women were also required to sign a form indicating they understood the medication’s risks. The FDA said Thursday there have been 26 deaths associated with the drug since 2000, though not all of those can be directly attributed to the medication due to underlying health conditions and other factors. Common drug side effects include cramping, bleeding, nausea, headache, and diarrhea. In some cases excess bleeding needs to be stopped with a surgical procedure. Near the beginning of the outbreak, the FDA waived in-person requirements for virtually all medications but left them in place for mifepristone. That triggered a lawsuit from the American College of Obstetricians and Gynecologists, which successfully overturned the restriction in federal court. The Trump administration then appealed the ruling to the Supreme Court, which reinstated the requirement in January. The point became moot — at least temporarily — in April when the FDA said it would not enforce the dispensing limits during the current public health emergency. “The FDA’s decision will come as a tremendous relief for countless abortion and miscarriage patients,” said Georgeanne Usova, a lawyer with the ACLU. “However, it is disappointing that the FDA fell short of repealing all of its medically unnecessary restrictions on mifepristone, and these remaining obstacles should also be lifted.” Jeanne Mancini, president of the March for Life Education and Defense Fund, said the decision “will lead to more lives lost to abortion, and will increase the number of mothers who suffer physical and psychological harm from chemical abortions.” Republished with the permission of the Associated Press.


